Partitioning of Catechol Derivatives in Lipid Membranes: Implications for Substrate Specificity to Catechol- O-methyltransferase
- PMID: 32101397
- PMCID: PMC7145343
- DOI: 10.1021/acschemneuro.0c00049
Partitioning of Catechol Derivatives in Lipid Membranes: Implications for Substrate Specificity to Catechol- O-methyltransferase
Abstract
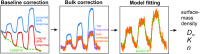
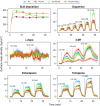
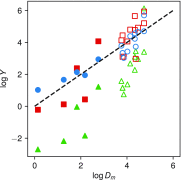
We have utilized multiparametric surface plasmon resonance and impendance-based quartz crystal microbalance instruments to study the distribution coefficients of catechol derivatives in cell model membranes. Our findings verify that the octanol-water partitioning coefficient is a poor descriptor of the total lipid affinity for small molecules which show limited lipophilicity in the octanol-water system. Notably, 3-methoxytyramine, the methylated derivative of the neurotransmitter dopamine, showed substantial affinity to the lipids despite its nonlipophilic nature predicted by octanol-water partitioning. The average ratio of distribution coefficients between 3-methoxytyramine and dopamine was 8.0. We also found that the interactions between the catechols and the membranes modeling the cell membrane outer leaflet are very weak, suggesting a mechanism other than the membrane-mediated mechanism of action for the neurotransmitters at the postsynaptic site. The average distribution coefficient for these membranes was one-third of the average value for pure phosphatidylcholine membranes, calculated using all compounds. In the context of our previous work, we further theorize that membrane-bound enzymes can utilize membrane headgroup partitioning to find their substrates. This could explain the differences in enzyme affinity between soluble and membrane-bound isoforms of catechol-O-methyltransferase, an essential enzyme in catechol metabolism.
Keywords: Catechols; distribution coefficient; multiparametric surface plasmon resonance; partition coefficient; quartz crystal microbalance; supported lipid bilayer.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Bennion B. J.; Be N. A.; McNerney M. W.; Lao V.; Carlson E. M.; Valdez C. A.; Malfatti M. A.; Enright H. A.; Nguyen T. H.; Lightstone F. C.; Carpenter T. S. (2017) Predicting a Drug’s Membrane Permeability: A Computational Model Validated with in Vitro Permeability Assay Data. J. Phys. Chem. B 121, 5228–5237. 10.1021/acs.jpcb.7b02914. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

