Neuropod Cells: The Emerging Biology of Gut-Brain Sensory Transduction
- PMID: 32101483
- PMCID: PMC7573801
- DOI: 10.1146/annurev-neuro-091619-022657
Neuropod Cells: The Emerging Biology of Gut-Brain Sensory Transduction
Abstract
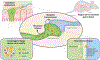
Guided by sight, scent, texture, and taste, animals ingest food. Once ingested, it is up to the gut to make sense of the food's nutritional value. Classic sensory systems rely on neuroepithelial circuits to convert stimuli into signals that guide behavior. However, sensation of the gut milieu was thought to be mediated only by the passive release of hormones until the discovery of synapses in enteroendocrine cells. These are gut sensory epithelial cells, and those that form synapses are referred to as neuropod cells. Neuropod cells provide the foundation for the gut to transduce sensory signals from the intestinal milieu to the brain through fast neurotransmission onto neurons, including those of the vagus nerve. These findings have sparked a new field of exploration in sensory neurobiology-that of gut-brain sensory transduction.
Keywords: enteroendocrine cells; glutamatergic transmission; gut-brain biology; neuropod cells; sensory transduction; vagus nerve.
Figures

References
-
- Altschuler SM, Ferenci DA, Lynn RB, Miselis RR. 1991. Representation of the cecum in the lateral dorsal motor nucleus of the vagus nerve and commissural subnucleus of the nucleus tractus solitarii in rat. J. Comp. Neurol 304:261–74 - PubMed
-
- Ball GG. 1974. Vagotomy: effect on electrically elicited eating and self-stimulation in the lateral hypothalamus. Science 184:484–85 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

