SST5 expression and USP8 mutation in functioning and silent corticotroph pituitary tumors
- PMID: 32101529
- PMCID: PMC7077525
- DOI: 10.1530/EC-20-0035
SST5 expression and USP8 mutation in functioning and silent corticotroph pituitary tumors
Abstract
Objective: Somatostatin receptor type 5 (SST5) is inconsistently expressed by corticotroph tumors, with higher expression found in corticotropinomas having ubiquitin-specific protease 8 (USP8) mutations. Aims were to study the correlation between characteristics of corticotropinomas and SST5 expression/USP8 mutation status and to describe the response to pasireotide in five patients.
Design: Retrospective cohort study.
Methods: Clinico-biochemical, radiological and pathological data of 62 patients, operated for a functioning or silent corticotropinoma between 2013 and 2017, were collected. SST5 expression was measured by immunohistochemistry (clone UMB-4, Abcam, IRS > 1 being considered positive), and Sanger sequencing was performed on 50 tumors to screen for USP8 mutations.
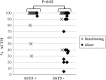
Results: SST5 expression was positive in 26/62 pituitary tumors. A moderate or strong IRS was found in 15/58 corticotropinomas and in 13/35 functioning corticotropinomas. Among functioning tumors, those expressing SST5 were more frequent in women (22/24 vs 9/15, P = 0.04) and had a lower grade (P = 0.04) compared to others. USP8 mutations were identified in 13/50 pituitary tumors and were more frequent in functioning compared to silent tumors (11/30 vs 2/20, P = 0.05). SST5 expression was more frequent in USP8mut vs USP8wt tumors (10/11 vs 7/19, P = 0.007). Among treated patients, normal urinary free cortisol levels were obtained in three patients (IRS 0, 2 and 6), while a four-fold decrease was observed in one patient (IRS 4).
Conclusion: SST5 expression appears to be associated with functioning, USP8mut and lower grade corticotropinomas. A correlation between SST5 expression or USP8mut and response to pasireotide remains to be confirmed.
Keywords: SST5; USP8; corticotroph pituitary tumors; pasireotide.
Figures


References
-
- van Haalen FM, Broersen LHA, Jorgensen JO, Pereira AM, Dekkers OM. Management of endocrine disease: mortality remains increased in Cushing’s disease despite biochemical remission: a systematic review and meta-analysis. European Journal of Endocrinology 2015. 172 R143–R149. (10.1530/EJE-14-0556) - DOI - PubMed
-
- Raverot G, Wierinckx A, Jouanneau E, Auger C, Borson-Chazot F, Lachuer J, Pugeat M, Trouillas J. Clinical, hormonal and molecular characterization of pituitary ACTH adenomas without (silent corticotroph adenomas) and with Cushing’s disease. European Journal of Endocrinology 2010. 163 35–43. (10.1530/EJE-10-0076) - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

