A C-terminal peptide from type I interferon protects the retina in a mouse model of autoimmune uveitis
- PMID: 32101556
- PMCID: PMC7043762
- DOI: 10.1371/journal.pone.0227524
A C-terminal peptide from type I interferon protects the retina in a mouse model of autoimmune uveitis
Abstract
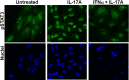
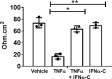
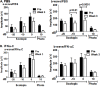
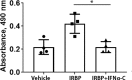
Experimental autoimmune uveitis (EAU) in rodents recapitulates many features of the disease in humans and has served as a useful tool for the development of therapeutics. A peptide from C-terminus of interferon α1, conjugated to palmitoyl-lysine for cell penetration, denoted as IFNα-C, was tested for its anti-inflammatory properties in ARPE-19 cells, followed by testing in a mouse model of EAU. Treatment with IFNα-C and evaluation by RT-qPCR showed the induction of anti-inflammatory cytokines and chemokine. Inflammatory markers induced by treatment with TNFα were suppressed when IFNα-C was simultaneously present. TNF-α mediated induction of NF-κB and signaling by IL-17A were attenuated by IFNα-C. Differentiated ARPE-19 cells were treated with TNFα in the presence or absence IFNα-C and analyzed by immmunhistochemistry. IFNα-C protected against the disruption integrity of tight junction proteins. Similarly, loss of transepithelial resistance caused by TNFα was prevented by IFNα-C. B10.RIII mice were immunized with a peptide from interphotoreceptor binding protein (IRBP) and treated by gavage with IFNα-C. Development of uveitis was monitored by histology, fundoscopy, SD-OCT, and ERG. Treatment with IFNα-C prevented uveitis in mice immunized with the IRBP peptide. Splenocytes isolated from mice with ongoing EAU exhibited antigen-specific T cell proliferation that was inhibited in the presence of IFNα-C. IFNα-C peptide exhibits anti-inflammatory properties and protects mice against damage to retinal structure and function suggesting that it has therapeutic potential for the treatment of autoimmune uveitis.
Conflict of interest statement
The University of Florida been awarded a patent (US 9,951,111B2) governing the interferon peptide mimetic used in this study. Two of the authors (CMA and HMJ) may obtain royalties from the licensing of this technology. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures




References
-
- Frohman LP. Treatment of Neuro-Ophthalmic Sarcoidosis. J Neuroophthalmol. 2014. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

