Two-step magnetic bead-based (2MBB) techniques for immunocapture of extracellular vesicles and quantification of microRNAs for cardiovascular diseases: A pilot study
- PMID: 32101583
- PMCID: PMC7043767
- DOI: 10.1371/journal.pone.0229610
Two-step magnetic bead-based (2MBB) techniques for immunocapture of extracellular vesicles and quantification of microRNAs for cardiovascular diseases: A pilot study
Abstract
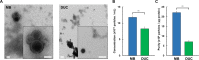
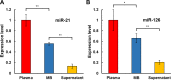
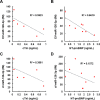
Extracellular vesicles (EVs) have attracted increasing attention because of their potential roles in various biological processes and medical applications. However, isolation of EVs is technically challenging mainly due to their small and heterogeneous size and contaminants that are often co-isolated. We have thus designed a two-step magnetic bead-based (2MBB) method for isolation a subset of EVs as well as their microRNAs from samples of a limited amount. The process involves utilizing magnetic beads coated with capture molecules that recognize EV surface markers, such as CD63. Captured EVs could be eluted from beads or lyzed directly for subsequent analysis. In this study, we used a second set of magnetic beads coated with complementary oligonucleotides to isolate EV-associated microRNAs (EV-miRNAs). The efficiencies of 2MBB processes were assessed by reverse transcription-polymerase chain reaction (RT-PCR) with spiked-in exogenous cel-miR-238 molecules. Experimental results demonstrated the high efficiency in EV enrichment (74 ± 7%, n = 4) and miRNA extraction (91 ± 4%, n = 4). Transmission electron micrographs (TEM) and nanoparticle tracking analysis (NTA) show that captured EVs enriched by 2MBB method could be released and achieved a higher purity than the differential ultracentrifugation (DUC) method (p < 0.001, n = 3). As a pilot study, EV-miR126-3p and total circulating cell-free miR126-3p (cf-miR126-3p) in eight clinical plasma samples were measured and compared with the level of protein markers. Compared to cf-miR126-3p, a significant increase in correlations between EV-miR126-3p and cardiac troponin I (cTnI) and N-terminal propeptide of B-type natriuretic peptide (NT-proBNP) was detected. Furthermore, EV-miR126-3p levels in plasma samples from healthy volunteers (n = 18) and high-risk cardiovascular disease (CVD) patients (n = 10) were significantly different (p = 0.006), suggesting EV-miR126 may be a potential biomarker for cardiovascular diseases. 2MBB technique is easy, versatile, and provides an efficient means for enriching EVs and EV-associated nucleic acid molecules.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
SIV Infection Regulates Compartmentalization of Circulating Blood Plasma miRNAs within Extracellular Vesicles (EVs) and Extracellular Condensates (ECs) and Decreases EV-Associated miRNA-128.Viruses. 2023 Feb 24;15(3):622. doi: 10.3390/v15030622. Viruses. 2023. PMID: 36992331 Free PMC article.
-
A magnetic bead-mediated selective adsorption strategy for extracellular vesicle separation and purification.Acta Biomater. 2021 Apr 1;124:336-347. doi: 10.1016/j.actbio.2021.02.004. Epub 2021 Feb 10. Acta Biomater. 2021. PMID: 33578055
-
Comparative analysis of extracellular vesicles miRNAs (EV-miRNAs) and cell-free microRNAs (cf-miRNAs) reveals that EV-miRNAs are more promising as diagnostic and prognostic biomarkers for prostate cancer.Gene. 2025 Mar 5;939:149186. doi: 10.1016/j.gene.2024.149186. Epub 2024 Dec 19. Gene. 2025. PMID: 39708932
-
Extracellular vesicles characteristics and emerging roles in atherosclerotic cardiovascular disease.Metabolism. 2018 Aug;85:213-222. doi: 10.1016/j.metabol.2018.04.008. Epub 2018 May 1. Metabolism. 2018. PMID: 29727628 Review.
-
Application of Extracellular Vesicles Proteomics to Cardiovascular Disease: Guidelines, Data Analysis, and Future Perspectives.Proteomics. 2019 Jan;19(1-2):e1800247. doi: 10.1002/pmic.201800247. Epub 2019 Jan 7. Proteomics. 2019. PMID: 30467982 Review.
Cited by
-
Immunoaffinity-enriched salivary small extracellular vesicles in periodontitis.Extracell Vesicles Circ Nucl Acids. 2023 Dec 30;4(4):698-712. doi: 10.20517/evcna.2023.48. eCollection 2023. Extracell Vesicles Circ Nucl Acids. 2023. PMID: 39697803 Free PMC article.
-
Extracellular Vesicle Capture by AnTibody of CHoice and Enzymatic Release (EV-CATCHER): A customizable purification assay designed for small-RNA biomarker identification and evaluation of circulating small-EVs.J Extracell Vesicles. 2021 Jun;10(8):e12110. doi: 10.1002/jev2.12110. Epub 2021 Jun 3. J Extracell Vesicles. 2021. PMID: 34122779 Free PMC article.
-
Fast quantification of extracellular vesicles levels in early breast cancer patients by Single Molecule Detection Array (SiMoA).Breast Cancer Res Treat. 2022 Feb;192(1):65-74. doi: 10.1007/s10549-021-06474-3. Epub 2021 Dec 21. Breast Cancer Res Treat. 2022. PMID: 34935096 Free PMC article.
-
Progress in Isolation and Molecular Profiling of Small Extracellular Vesicles via Bead-Assisted Platforms.Biosensors (Basel). 2023 Jun 28;13(7):688. doi: 10.3390/bios13070688. Biosensors (Basel). 2023. PMID: 37504087 Free PMC article. Review.
-
EV-Elute: A universal platform for the enrichment of functional surface marker-defined extracellular vesicle subpopulations.J Extracell Vesicles. 2024 Dec;13(12):e70017. doi: 10.1002/jev2.70017. J Extracell Vesicles. 2024. PMID: 39692115 Free PMC article.
References
-
- Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1). 10.1080/20013078.2018.1535750 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

