Quantile-dependent expressivity of postprandial lipemia
- PMID: 32101585
- PMCID: PMC7043740
- DOI: 10.1371/journal.pone.0229495
Quantile-dependent expressivity of postprandial lipemia
Abstract
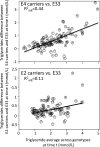
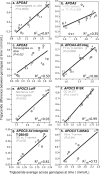
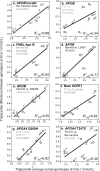
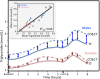
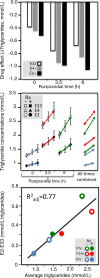
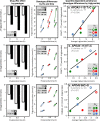
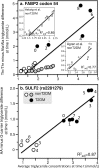
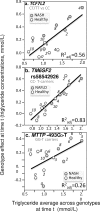
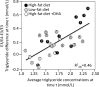
Purpose: "Quantile-dependent expressivity" describes an effect of the genotype that depends upon the level of the phenotype (e.g., whether a subject's triglycerides are high or low relative to its population distribution). Prior analyses suggest that the effect of a genetic risk score (GRS) on fasting plasma triglyceride levels increases with the percentile of the triglyceride distribution. Postprandial lipemia is well suited for testing quantile-dependent expressivity because it exposes each individual's genotype to substantial increases in their plasma triglyceride concentrations. Ninety-seven published papers were identified that plotted mean triglyceride response vs. time and genotype, which were converted into quantitative data. Separately, for each published graph, standard least-squares regression analysis was used to compare the genotype differences at time t (dependent variable) to average triglyceride concentrations at time t (independent variable) to assess whether the genetic effect size increased in association with higher triglyceride concentrations and whether the phenomenon could explain purported genetic interactions with sex, diet, disease, BMI, and drugs.
Results: Consistent with the phenomenon, genetic effect sizes increased (P≤0.05) with increasing triglyceride concentrations for polymorphisms associated with ABCA1, ANGPTL4, APOA1, APOA2, APOA4, APOA5, APOB, APOC3, APOE, CETP, FABP2, FATP6, GALNT2, GCKR, HL, IL1b, LEPR, LOX-1, LPL, MC4R, MTTP, NPY, SORT1, SULF2, TNFA, TCF7L2, and TM6SF2. The effect size for these polymorphisms showed a progressively increasing dose-response, with intermediate effect sizes at intermediate triglyceride concentrations. Quantile-dependent expressivity provided an alternative interpretation to their interactions with sex, drugs, disease, diet, and age, which have been traditionally ascribed to gene-environment interactions and genetic predictors of drug efficacy (i.e., personalized medicine).
Conclusion: Quantile-dependent expressivity applies to the majority of genetic variants affecting postprandial triglycerides, which may arise because the impaired functionalities of these variants increase at higher triglyceride concentrations. Purported gene-drug interactions may be the manifestations of quantile-dependent expressivity, rather than genetic predictors of drug efficacy.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

