Cu-Catalyzed Diastereo- and Enantioselective Reactions of γ,γ-Disubstituted Allyldiboron Compounds with Ketones
- PMID: 32101637
- PMCID: PMC7230022
- DOI: 10.1002/anie.202000675
Cu-Catalyzed Diastereo- and Enantioselective Reactions of γ,γ-Disubstituted Allyldiboron Compounds with Ketones
Abstract
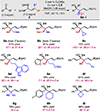
A catalytic diastereo- and enantioselective method for the preparation of complex tertiary homoallylic alcohols containing a vicinal quaternary carbon stereogenic center and a versatile alkenylboronic ester is disclosed. Transformations are promoted by 5 mol % of a readily available copper catalyst bearing a bulky monodentate phosphoramidite ligand, which is essential for attaining both high dr and er. Reactions proceed with a wide variety of ketones and allylic 1,1-diboronate reagents, which enables the efficient preparation of diverse array of molecular scaffolds.
Keywords: 1,1-allyldiboron compounds; asymmetric catalysis; copper; ketones; quaternary carbon centers.
© 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures




References
-
-
For recent reviews on the enantioselective synthesis of quaternary carbon stereogenic centers, see:
Das JP, Marek I, Chem. Commun 2011, 47, 4593–4623.Minko Y, Marek I, Chem. Commun 2014, 50, 12597–12611.Marek I, Minko Y, Pasco M, Mejuch T, Gilboa N, Chechik H, Das JP, J. Am. Chem. Soc 2014, 136, 2682–2694.Quasdorf KW, Overman LE, Nature 2014, 516, 181–191.Liu Y, Han S-J, Liu W-B, Stoltz BM, Acc. Chem. Res 2015, 48, 740–751.Zeng X-P, Cao Z-Y, Wang Y-H, Zhou F, Zhou J, Chem. Rev 2016, 116, 7330–7396.Feng J, Holmes M, Krische MJ, Chem. Rev 2017, 117, 12564–12580.
-
-
-
For recent reviews on the enantioselective synthesis of tertiary alcohols, see:
Riant O, Hannedouche J, Org. Biomol. Chem 2007, 5, 873–888.Hatano M, Ishihara K, Synthesis 2008, 1647–1675.Shibasaki M, Kanai M, Chem. Rev 2008, 108, 2853–2873.(d) Liu Y-L, Lin X-T, Adv. Synth. Catal 2019, 361, 876–918.
-
-
-
For a recent reviews of catalytic allyl additions to ketones, see:
Denmark SE, Fu J, Chem. Rev 2003, 103, 2763–2794.Shibasaki M, Kanai M, Chem. Rev 2008, 108, 2853–2873.Yus M, Gómez González- J. C., Foubelo F, Chem. Rev 2011, 111, 7774–7854.Huo H-X, Duvall JR, Huang M-Y, Hong R, Org. Chem. Front 2014, 1, 303–320. See also ref. 1g.
-
-
-
For representative catalytic enantioselective examples of crotyl additions to ketones, see:
Wada R, Oisaki K, Kanai M, Shibasaki M, J. Am. Chem. Soc 2004, 126, 8910–8911.Wadamoto M, Yamamoto H, J. Am. Chem. Soc 2005, 127, 14556–14557.Miller JJ, Sigman MS, J. Am. Chem. Soc 2007, 129, 2752–2753.Kanai M, Wada R, Shibuguchi T, Shibasaki M, Pure Appl. Chem 2008, 80, 1055–1062.Schneider U, Ueno M, Kobayashi S, J. Am. Chem. Soc. 2008, 130, 13824–13825.Rauniyar V, Zhai H, Hall DG, J. Am. Chem. Soc 2008, 130, 8481–8490.Barnett DS, Moquist PN, Schaus SE, Angew. Chem. Int. Ed 2009, 48, 8679–8682; Angew. Chem. 2009, 121, 8835–8838.Wadamoto M, Naodovic M, Yamamoto H, Eur. J. Org. Chem 2009, 5132–5134.Nowrouzi F, Thadani AN, Batey RA, Org. Lett. 2009, 11, 2631–2634.Shi S-L, Xu L-W, Oisaki K, Kanai M, Shibasaki M, J. Am. Chem. Soc 2010, 132, 6638–6639.Yamaguchi M, Morita N, Scheider U, Kobayashi S, Adv. Synth. Catal 2010, 352, 1461–1465.Tsai EY, Liu RY, Yang Y, Buchwald SL, J. Am. Chem. Soc 2018, 140, 2007–2011.Li C, Liu RY, Jesikiewicz LT, Yang Y, Liu P, Buchwald SL, J. Am. Chem. Soc 2019, 141, 5062–5070.
-
-
-
For examples of enantioselective allyl additions to aldehydes that generate quaternary carbon stereogenic centers, see:
Denmark SE, Fu J, J. Am. Chem. Soc 2001, 123, 9488–9489.Denmark SE, Fu J, Org. Lett 2002, 4, 1951–1953.Feng J, Garza VJ, Krische MJ, J. Am. Chem. Soc 2014, 136, 8911–8914.Nguyen KD, Herkommer D, Krische MJ, J. Am. Chem. Soc 2016, 138, 14210–14213.Holmes M, Nguyen KD, Schwartz LA, Luong T, Krische MJ, J. Am. Chem. Soc 2017, 139, 8114–8117.Schwartz LA, Holmes M, Brito GA, Gonçalves TP, Richardson J, Ruble JC, Huang K-W, Krische MJ, J. Am. Chem. Soc 2019, 141, 2087–2096.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

