Dysbiosis-Induced Secondary Bile Acid Deficiency Promotes Intestinal Inflammation
- PMID: 32101703
- PMCID: PMC8172352
- DOI: 10.1016/j.chom.2020.01.021
Dysbiosis-Induced Secondary Bile Acid Deficiency Promotes Intestinal Inflammation
Abstract
Secondary bile acids (SBAs) are derived from primary bile acids (PBAs) in a process reliant on biosynthetic capabilities possessed by few microbes. To evaluate the role of BAs in intestinal inflammation, we performed metabolomic, microbiome, metagenomic, and transcriptomic profiling of stool from ileal pouches (surgically created resevoirs) in colectomy-treated patients with ulcerative colitis (UC) versus controls (familial adenomatous polyposis [FAP]). We show that relative to FAP, UC pouches have reduced levels of lithocholic acid and deoxycholic acid (normally the most abundant gut SBAs), genes required to convert PBAs to SBAs, and Ruminococcaceae (one of few taxa known to include SBA-producing bacteria). In three murine colitis models, SBA supplementation reduces intestinal inflammation. This anti-inflammatory effect is in part dependent on the TGR5 bile acid receptor. These data suggest that dysbiosis induces SBA deficiency in inflammatory-prone UC patients, which promotes a pro-inflammatory state within the intestine that may be treated by SBA restoration.
Keywords: bile acids; colitis; dysbiosis; inflammatory bowel disease; metabolomics; pouchitis; ulcerative colitis.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests All authors declare no competing interests.
Figures
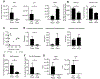


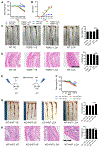
References
-
- ANDERSON MJ 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology, 26, 32–46.
-
- BANKS C, BATEMAN A, PAYNE R, JOHNSON P & SHERON N 2003. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn’s disease. J Pathol, 199, 28–35. - PubMed
-
- BEHR C, SPERBER S, JIANG X, STRAUSS V, KAMP H, WALK T, HEROLD M, BEEKMANN K, RIETJENS I & VAN RAVENZWAAY B 2018. Microbiome-related metabolite changes in gut tissue, cecum content and feces of rats treated with antibiotics. Toxicol Appl Pharmacol, 355, 198–210. - PubMed
-
- BUFFIE CG, BUCCI V, STEIN RR, MCKENNEY PT, LING L, GOBOURNE A, NO D, LIU H, KINNEBREW M, VIALE A, LITTMANN E, VAN DEN BRINK MR, JENQ RR, TAUR Y, SANDER C, CROSS JR, TOUSSAINT NC, XAVIER JB & PAMER EG 2015. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature, 517, 205–8. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

