Type I Interferons Suppress Anti-parasitic Immunity and Can Be Targeted to Improve Treatment of Visceral Leishmaniasis
- PMID: 32101732
- PMCID: PMC7981274
- DOI: 10.1016/j.celrep.2020.01.099
Type I Interferons Suppress Anti-parasitic Immunity and Can Be Targeted to Improve Treatment of Visceral Leishmaniasis
Abstract
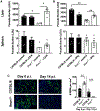
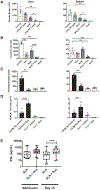
Type I interferons (IFNs) play critical roles in anti-viral and anti-tumor immunity. However, they also suppress protective immune responses in some infectious diseases. Here, we identify type I IFNs as major upstream regulators of CD4+ T cells from visceral leishmaniasis (VL) patients. Furthermore, we report that mice deficient in type I IFN signaling have significantly improved control of Leishmania donovani, a causative agent of human VL, associated with enhanced IFNγ but reduced IL-10 production by parasite-specific CD4+ T cells. Importantly, we identify a small-molecule inhibitor that can be used to block type I IFN signaling during established infection and acts synergistically with conventional anti-parasitic drugs to improve parasite clearance and enhance anti-parasitic CD4+ T cell responses in mice and humans. Thus, manipulation of type I IFN signaling is a promising strategy for improving disease outcome in VL patients.
Keywords: CD4(+) T cells; type I interferons; visceral leishmaniasis.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests S.W.L. has served on an advisory board for Novartis, the owner of Jakavi (ruxolitinib), a drug used in this study.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

