High-Accuracy Detection of Neuronal Ensemble Activity in Two-Photon Functional Microscopy Using Smart Line Scanning
- PMID: 32101736
- PMCID: PMC7043026
- DOI: 10.1016/j.celrep.2020.01.105
High-Accuracy Detection of Neuronal Ensemble Activity in Two-Photon Functional Microscopy Using Smart Line Scanning
Abstract
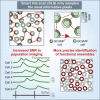
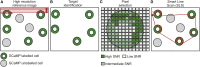
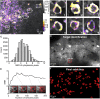
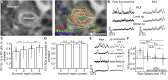
Two-photon functional imaging using genetically encoded calcium indicators (GECIs) is one prominent tool to map neural activity. Under optimized experimental conditions, GECIs detect single action potentials in individual cells with high accuracy. However, using current approaches, these optimized conditions are never met when imaging large ensembles of neurons. Here, we developed a method that substantially increases the signal-to-noise ratio (SNR) of population imaging of GECIs by using galvanometric mirrors and fast smart line scan (SLS) trajectories. We validated our approach in anesthetized and awake mice on deep and dense GCaMP6 staining in the mouse barrel cortex during spontaneous and sensory-evoked activity. Compared to raster population imaging, SLS led to increased SNR, higher probability of detecting calcium events, and more precise identification of functional neuronal ensembles. SLS provides a cheap and easily implementable tool for high-accuracy population imaging of neural GCaMP6 signals by using galvanometric-based two-photon microscopes.
Keywords: GCaMP6; barrel cortex; neuronal ensembles; spatiotemporal neural responses; two-photon imaging.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures




Similar articles
-
Multiphoton minimal inertia scanning for fast acquisition of neural activity signals.J Neural Eng. 2018 Apr;15(2):025003. doi: 10.1088/1741-2552/aa99e2. J Neural Eng. 2018. PMID: 29129832
-
Automated correction of fast motion artifacts for two-photon imaging of awake animals.J Neurosci Methods. 2009 Jan 15;176(1):1-15. doi: 10.1016/j.jneumeth.2008.08.020. Epub 2008 Aug 26. J Neurosci Methods. 2009. PMID: 18789968
-
GCaMP as an indirect measure of electrical activity in rat trigeminal ganglion neurons.Cell Calcium. 2020 Jul;89:102225. doi: 10.1016/j.ceca.2020.102225. Epub 2020 May 30. Cell Calcium. 2020. PMID: 32505783 Free PMC article.
-
Voltage imaging with ANNINE dyes and two-photon microscopy of Purkinje dendrites in awake mice.Neurosci Res. 2020 Mar;152:15-24. doi: 10.1016/j.neures.2019.11.007. Epub 2019 Nov 20. Neurosci Res. 2020. PMID: 31758973 Review.
-
Probing Deep Brain Circuitry: New Advances in in Vivo Calcium Measurement Strategies.ACS Chem Neurosci. 2017 Feb 15;8(2):243-251. doi: 10.1021/acschemneuro.6b00307. Epub 2017 Feb 2. ACS Chem Neurosci. 2017. PMID: 27984692 Review.
Cited by
-
SmaRT2P: a software for generating and processing smart line recording trajectories for population two-photon calcium imaging.Brain Inform. 2022 Aug 4;9(1):18. doi: 10.1186/s40708-022-00166-4. Brain Inform. 2022. PMID: 35927517 Free PMC article.
-
Optogenetic strategies for high-efficiency all-optical interrogation using blue-light-sensitive opsins.Elife. 2021 May 25;10:e63359. doi: 10.7554/eLife.63359. Elife. 2021. PMID: 34032211 Free PMC article.
-
A deep-learning approach for online cell identification and trace extraction in functional two-photon calcium imaging.Nat Commun. 2022 Mar 22;13(1):1529. doi: 10.1038/s41467-022-29180-0. Nat Commun. 2022. PMID: 35318335 Free PMC article.
-
Aberration correction in long GRIN lens-based microendoscopes for extended field-of-view two-photon imaging in deep brain regions.Elife. 2025 May 2;13:RP101420. doi: 10.7554/eLife.101420. Elife. 2025. PMID: 40314426 Free PMC article.
-
Fluorescence imaging of large-scale neural ensemble dynamics.Cell. 2022 Jan 6;185(1):9-41. doi: 10.1016/j.cell.2021.12.007. Cell. 2022. PMID: 34995519 Free PMC article. Review.
References
-
- Akemann W., Léger J.F., Ventalon C., Mathieu B., Dieudonné S., Bourdieu L. Fast spatial beam shaping by acousto-optic diffraction for 3D non-linear microscopy. Opt. Express. 2015;23:28191–28205. - PubMed
-
- Bindocci E., Savtchouk I., Liaudet N., Becker D., Carriero G., Volterra A. Three-dimensional Ca2+ imaging advances understanding of astrocyte biology. Science. 2017;356:eaai8185. - PubMed
-
- Bovetti S., Fellin T. Optical dissection of brain circuits with patterned illumination through the phase modulation of light. J. Neurosci. Methods. 2015;241:66–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

