Effectiveness of Switching to Darunavir/Cobicistat in Virologically Suppressed HIV-Positive Patients Receiving Ritonavir-Boosted Protease Inhibitor-Based Regimen: The "STORE" Study
- PMID: 32101882
- PMCID: PMC7289135
- DOI: 10.1097/QAI.0000000000002331
Effectiveness of Switching to Darunavir/Cobicistat in Virologically Suppressed HIV-Positive Patients Receiving Ritonavir-Boosted Protease Inhibitor-Based Regimen: The "STORE" Study
Abstract
Objective: This study investigates the effectiveness and tolerability of switching to a darunavir/cobicistat (DRV/c)-based antiretroviral regimen from a ritonavir-boosted protease inhibitor (PI/r)-based regimen in virologically suppressed HIV-positive patients. DRV trough values were also investigated.
Setting: Prospective, multicenter, single-country, noninterventional cohort study.
Methods: This study included patients on a PI/r-based ART for at least 12 months having plasma HIV-1 RNA <50 copies/mL since at least 6 months. The primary endpoint, defined as HIV-1 RNA <50 copies/mL, was measured at 48 ± 6 weeks from baseline. A secondary analysis was performed using the time to loss of virological response algorithm. Biochemical parameters, including DRV trough samples, were collected as per clinical practice and measured using high-performance liquid chromatography.
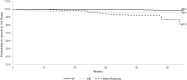
Results: Of 336 patients enrolled, 282 completed the study: 70.8% had plasma HIV-1 RNA <50 copies/mL at 48 weeks; using the time to loss of virological response algorithm, 82.7% maintained virological suppression. Virological failure was observed in 6 patients (1.8%). Adverse event-related discontinuations were 4.5%. After 48 weeks, we found a significant improvement in both triglycerides (median, 130 to 113.5 mg/dL, P = 0.0254) and high-density lipoprotein cholesterol (48 to 49 mg/dL, P < 0.0001) but no change in other biomarkers. DRV trough concentrations in 56 subjects showed a median value of 2862.5 (1469.5-4439) ng/mL, higher in women than in men (4221 vs. 2634 ng/mL, P = 0.046).
Conclusions: In stable HIV-1 positive virologically suppressed patients, the switch to DRV/c-based ART was beneficial in terms of low rates of virological failure and adverse events due to its high tolerability and improvement in triglycerides.
Conflict of interest statement
A.G. received a grant from Janssen-Cilag, ViiV Healthcare, MSD, BMS, Abbvie, Gilead Sciences, Novartis, Pfizer, Astellas, AstraZeneca and Angelini; he received consulting fees or honorarium from Janssen-Cilag, ViiV Healthcare, MSD, BMS, Abbvie, Gilead Sciences, Novartis, Pfizer, Astellas, AstraZeneca and Angelini. A.A. received a grant from Gilead Sciences, ViiV Healthcare and Janssen-Cilag; he received consulting fees or honorarium from ViiV Healthcare, Janssen-Cilag and Merck. S.R. received grants research support, compensation for CME activities or advisory boards form ViiV Healthcare, MSD, Gilead Sciences and Janssen. S.B. has received grants, travel grants and consultancy fees from Abbvie, BMS, MSD, Gilead, Janssen-Cilag, and ViiV. A.U., R.T., and D.M. are Janssen employees. The remaining authors have no conflicts of interest to disclose.
Figures
References
-
- Curran A, Pérez-Valero I, Moltó J. Rezolsta® (darunavir/cobicistat): first boosted protease inhibitor co-formulated with cobicistat. AIDS Rev. 2015;17:114–120. - PubMed
-
- Capetti A, Cossu MV, Rizzardini G. Darunavir/cobicistat for the treatment of HIV-1: a new era for compact drugs with high genetic barrier to resistance. Expert Opin Pharmacother. 2015;16:2689–2702. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous