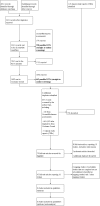
Biologics for chronic rhinosinusitis
- PMID: 32102112
- PMCID: PMC7043934
- DOI: 10.1002/14651858.CD013513.pub2
Biologics for chronic rhinosinusitis
Update in
-
Biologics for chronic rhinosinusitis.Cochrane Database Syst Rev. 2021 Mar 12;3(3):CD013513. doi: 10.1002/14651858.CD013513.pub3. Cochrane Database Syst Rev. 2021. PMID: 33710614 Free PMC article.
Abstract
Background: This living systematic review is one of several Cochrane Reviews evaluating the medical management of patients with chronic rhinosinusitis. Chronic rhinosinusitis is common. It is characterised by inflammation of the nasal and sinus linings, nasal blockage, rhinorrhoea, facial pressure/pain and loss of sense of smell. It occurs with or without nasal polyps. 'Biologics' are medicinal products produced by a biological process. Monoclonal antibodies are one type, already evaluated in related inflammatory conditions (e.g. asthma and atopic dermatitis).
Objectives: To assess the effects of biologics for the treatment of chronic rhinosinusitis.
Search methods: The Cochrane ENT Information Specialist searched the Cochrane ENT Register; CENTRAL (2019, Issue 9); Ovid MEDLINE; Ovid Embase; Web of Science; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 16 September 2019.
Selection criteria: Randomised controlled trials (RCTs) with at least three months follow-up comparing biologics (currently, monoclonal antibodies) against placebo/no treatment in patients with chronic rhinosinusitis.
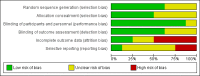
Data collection and analysis: We used standard Cochrane methodological procedures. Our primary outcomes were disease-specific health-related quality of life (HRQL), disease severity and serious adverse events (SAEs). The secondary outcomes were avoidance of surgery, extent of disease (measured by endoscopic or computerised tomography (CT) score), generic HRQL and adverse events (nasopharyngitis, including sore throat). We used GRADE to assess the certainty of the evidence for each outcome.
Main results: We included eight RCTs. Of 986 adult participants, 984 had severe chronic rhinosinusitis with nasal polyps; 43% to 100% of participants also had asthma. Three biologics, with different targets, were evaluated: dupilumab, mepolizumab and omalizumab. All the studies were sponsored or supported by industry. Anti-IL-4Rα mAb (dupilumab) versusplacebo/no treatment (all receiving intranasal steroids) Three studies (784 participants) evaluated dupilumab. Disease-specific HRQL was measured with the SNOT-22 (score 0 to 110; minimal clinically important difference (MCID) 8.9 points). At 24 weeks, the SNOT-22 score was 19.61 points lower (better) in participants receiving dupilumab (mean difference (MD) -19.61, 95% confidence interval (CI) -22.54 to -16.69; 3 studies; 784 participants; high certainty). Symptom severity measured on a 0- to 10-point visual analogue scale (VAS) was 3.00 lower in those receiving dupilumab (95% CI -3.47 to -2.53; 3 studies; 784 participants; moderate certainty). The risk of serious adverse events may be lower in the dupilumab group (risk ratio (RR) 0.45, 95% CI 0.28 to 0.75; 3 studies; 782 participants; low certainty). The number of participants requiring nasal polyp surgery (actual or planned) during the treatment period is probably lower in those receiving dupilumab (RR 0.17, 95% CI 0.05 to 0.52; 2 studies; 725 participants; moderate certainty). Change in the extent of disease using the Lund Mackay computerised tomography (CT) score (0 to 24, higher = worse) was -7.00 (95% CI -9.61 to -4.39; 3 studies; 784 participants; high certainty), a large effect favouring the dupilumab group. The EQ-5D visual analogue scale (0 to 100, higher = better; MCID 8 points) was used to measure change in generic quality of life. The mean difference favouring dupilumab was 8.59 (95% CI 5.31 to 11.86; 2 studies; 706 participants; moderate certainty). There may be little or no difference in the risk of nasopharyngitis (RR 0.95, 95% CI 0.72 to 1.25; 3 studies; 783 participants; low certainty). Anti-IL-5 mAb (mepolizumab) versusplacebo/no treatment (all receiving intranasal steroids) Two studies (137 participants) evaluated mepolizumab. Disease-specific HRQL measured with the SNOT-22 at 25 weeks was 13.26 points lower (better) in participants receiving mepolizumab (95% CI -22.08 to -4.44; 1 study; 105 participants; low certainty; MCID 8.9). It is very uncertain whether there is a difference in s ymptom severity: on a 0- to 10-point VAS symptom severity was -2.03 lower in those receiving mepolizumab (95% CI -3.65 to -0.41; 1 study; 72 participants; very low certainty). It is very uncertain if there is difference in the risk of serious adverse events (RR 1.57, 95% CI 0.07 to 35.46; 2 studies; 135 participants, very low certainty). It is very uncertain whether or not the overall risk that patients still need surgery at trial end is lower in the mepolizumab group (RR 0.78, 95% CI 0.64 to 0.94; 2 studies; 135 participants; very low certainty). It is very uncertain whether mepolizumab reduces the extent of disease as measured by endoscopic nasal polyps score (scale range 0 to 8). The mean difference was 1.23 points lower in the mepolizumab group (MD -1.23, 95% -1.79 to -0.68; 2 studies; 137 participants; very low certainty). The difference in generic quality of life (EQ-5D) was 5.68 (95% CI -1.18 to 12.54; 1 study; 105 participants; low certainty), favouring the mepolizumab group. This difference is smaller than the MCID of 8 points. There may be little or no difference in the risk of nasopharyngitis (RR 0.73, 95% 0.36 to 1.47; 2 studies; 135 participants; low certainty). Anti-IgE mAb (omalizumab) versus placebo/no treatment (all receiving intranasal steroids) Three very small studies (65 participants) evaluated omalizumab. We are very uncertain about the effect of omalizumab on disease-specific HRQL, severe adverse events, extent of disease (CT scan scores), generic HRQL and adverse effects.
Authors' conclusions: In adults with severe chronic rhinosinusitis and nasal polyps, using regular topical nasal steroids, dupilumab improves disease-specific HRQL compared to placebo, and reduces the extent of the disease as measured on a CT scan. It probably also improves symptoms and generic HRQL and there is no evidence of an increased risk of serious adverse events. It may reduce the need for further surgery. There may be little or no difference in the risk of nasopharyngitis. In similar patients, mepolizumab may improve both disease-specific and generic HRQL. It is uncertain whether it reduces the need for surgery or improves nasal polyp scores. There may be little or no difference in the risk of nasopharyngitis. It is uncertain if there is a difference in symptom severity and the risk of serious adverse events. We are uncertain about the effects of omalizumab.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Lee‐Yee Chong: none known.
Patorn Piromchai: none known.
Steve Sharp: Steve Sharp's employer, the National Institute for Health and Care Excellence (NICE), has produced guidance on related topics such as sinusitis, which he has not contributed to.
Kornkiat Snidvongs: none known.
Carl Philpott: Carl Philpott has previously received consultancy fees for Acclarent, Navigant, Aerin Medical and Entellus, and is a trustee of the patient charity Fifth Sense. He is an investigator on a clinical trial that may be included in this review, but will have no role in the data extraction, risk of bias assessment or data analysis for this study.
Claire Hopkins: Claire Hopkins has participated in advisory boards for Olympus, Chordate, Smith & Nephew and Sanofi to provide expertise with regards to study design and outcome assessment, and interpretation of trial data. She is an investigator on a clinical trial that is included in this review, but had no role in the data extraction, risk of bias assessment or data analysis for this study (LIBERTY SINUS 24; LIBERTY SINUS 52).
Martin J Burton: Professor Martin Burton is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review.
Figures
References
References to studies included in this review
Bachert 2016 {published and unpublished data}
-
- Bachert C, Hellings P, Mullol J, Hamilos D, Naclerio R, Joish VN, et al. Dupilumab improves patient‐reported outcomes in chronic sinusitis with nasal polyps patients with comorbid asthma: results from a phase 2a trial. European Respiratory Journal 2016;48(Suppl 60):OA251. [5632585]
-
- Bachert C, Hellings PW, Mullol J, Hamilos DL, Gevaert P, Naclerio RM, et al. Dupilumab improves health‐related quality of life in patients with chronic rhinosinusitis with nasal polyposis. Allergy 2019;71:12. [PUBMED: 31306495] - PubMed
-
- Bachert C, Hellings PW, Mullol J, Naclerio RM, Hamilos DL, Gevaert P, et al. Atopic comorbidities and biomarkers of type 2 inflammation in patients with chronic rhinosinusitis with nasal polyposis (CRSwNP) who failed intranasal corticosteroids. Journal of Allergy and Clinical Immunology 2018;141(2 Suppl 1):AB90. [7912807]
-
- Bachert C, Mannent L, Naclerio RM, Mullol J, Ferguson BJ, Gevaert P, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA 2016;315(5):469‐79. [1861626; PUBMED: 26836729] - PubMed
-
- Bachert C, Mannent L, Naclerio RM, Mullol J, Ferguson BJ, Jiao L, et al. Dupilumab in chronic sinusitis with nasal polyposis, with and without asthma. Allergy 2015;70:107. [1866543]
Bachert 2017 {published and unpublished data}
-
- Bachert C, Sousa AR, Lund VJ, Scadding GK, Gevaert P, Nasser S, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: randomized trial. Journal of Allergy and Clinical Immunology 2017;140(4):1024‐31.e14. [7111780; PUBMED: 28687232] - PubMed
-
- EUCTR2008‐003772‐21‐NL. A two‐part, randomised, double‐blind, placebo controlled, multi‐center study to investigate the use of mepolizumab (SB‐240563) in reducing the need for surgery in subjects with severe bilateral nasal polyposis. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2008‐003772‐21/NL (first received 27 February 2009). [1743659]
-
- GlaxoSmithKline. A randomised, double blind, placebo controlled, multi‐centre study to investigate the use of mepolizumab (SB240563) in reducing the need for surgery in subjects with severe bilateral nasal polyposis. GSK Clinical Study Report. [MPP111782]
-
- NCT01362244. A randomised, double‐blind, placebo controlled, multi‐center study to investigate the use of mepolizumab (Sb‐240563) in reducing the need for surgery in subjects with severe bilateral nasal polyposis. https://clinicaltrials.gov/show/nct01362244 (first received 26 May 2011). [1629203]
Gevaert 2011 {published data only}
Gevaert 2013 {published and unpublished data}
-
- EUCTR2006‐003524‐11‐BE. Clinical and biological effects of anti‐IgE (omalizumab) in patients with bilateral nasal polyposis and asthma [A randomized, double‐blind, phase 2, placebo controlled, 2 arm study to evaluate dupilumab in patients with bilateral nasal polyposis and chronic symptoms of sinusitis]. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2006‐003524‐11‐BE (first received 11 August 2006). [1770331]
-
- Gevaert P, Calus L, Zele T, Blomme K, Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and non‐allergic patients with nasal polyps and asthma. Journal of Allergy and Clinical Immunology 2012;129(2 Suppl 1):AB69. [1765640] - PubMed
-
- Gevaert P, Calus L, Zele T, Blomme K, Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. Journal of Allergy and Clinical Immunology 2013;131(1):110‐6.e1. [1617401; PUBMED: 23021878] - PubMed
-
- NCT01393340. Clinical and biological effects of anti‐IgE (omalizumab) in patients with bilateral nasal polyposis and asthma. https://clinicaltrials.gov/show/NCT01393340 (first received 13 July 2011). [1629212]
LIBERTY SINUS 24 {published and unpublished data}
-
- Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS‐24 and LIBERTY NP SINUS‐52): results from two multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group phase 3 trials. Lancet 2019;394(10209):1638‐50. [12131774; PUBMED: 31543428] - PubMed
-
- EUCTR2015‐003101‐42‐BG. Controlled clinical study of dupilumab in patients with nasal polyps [A randomized, 24‐week treatment, double‐blind, placebo‐controlled efficacy and safety study of dupilumab 300 mg every other week, in patients with bilateral nasal polyposis on a background therapy with intranasal corticosteroids ‐ SYNUS‐24]. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_nu... (first received 4 January 2017). [10716209]
-
- Han JK, Bachert C, Desrosiers M, Laidlaw TM, Hopkins C, Fokkens WJ, et al. Efficacy and safety of dupilumab in patients with chronic rhinosinusitis with nasal polyps: results from the randomized phase 3 sinus‐24 study. Journal of Allergy and Clinical Immunology 2019;143(2):AB422. [10639235]
-
- NCT02912468. A controlled clinical study of dupilumab in patients with bilateral nasal polyps [A randomized, 24‐week treatment, double‐blind, placebo‐controlled efficacy and safety study of dupilumab 300 mg every other week, in patients with bilateral nasal polyposis on a background therapy with intranasal corticosteroids]. https://clinicaltrials.gov/show/NCT02912468 (first received 23 September 2016). [6978608]
LIBERTY SINUS 52 {published and unpublished data}
-
- Bachert C, Desrosiers M, Mullol J, Hellings PW, Cervin A, Sher L, et al. A randomized phase 3 study, sinus‐52, evaluating the efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps. Journal of Allergy and Clinical Immunology 2019;143(2):AB433. [9965830]
-
- Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS‐24 and LIBERTY NP SINUS‐52): results from two multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group phase 3 trials. Lancet 2019;394(10209):1638‐50. [12131774; PUBMED: 31543428] - PubMed
-
- Bachert C, Hellings PW, Mullol J, Naclerio RM, Chao J, Amin N, et al. Dupilumab improves patient‐reported outcomes in patients with chronic rhinosinusitis with nasal polyps and comorbid asthma. Journal of Allergy and Clinical Immunology: In Practice 2019;7(7):2447‐9.e2. [12043116; PUBMED: 30928658] - PubMed
-
- EUCTR2015‐001314‐10‐ES. Controlled clinical study of dupilumab in patients with nasal polyps [A randomized, double‐blind, 52‐week, placebo controlled efficacy and safety study of dupilumab, in patients with bilateral nasal polyposis on a background therapy with intranasal corticosteroids]. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_nu... (first received 10 October 2016). [10715675]
-
- NCT02898454. Controlled clinical study of dupilumab in patients with nasal polyps [A randomized, double‐blind, 52‐week, placebo controlled efficacy and safety study of dupilumab, in patients with bilateral nasal polyposis on a background therapy with intranasal corticosteroids]. https://clinicaltrials.gov/show/NCT02898454 (first received 13 September 2016). [6978611]
NCT01066104 {unpublished data only}
-
- NCT01066104. Subcutaneous omalizumab for treatment of chronic rhinosinusitis with nasal polyposis. https://clinicaltrials.gov/show/nct01066104 2009. [CRS: 1647960]
Pinto 2010 {published data only}
-
- Mehta NJ, Pinto J, Tineo M, Baroody FM, Naclerio RM. A randomized, double‐blind, placebo‐controlled clinical trial of omalizumab for chronic rhinosinusitis. Journal of Allergy and Clinical Immunology 2009;123(2 Suppl 1):S201. [CRS: 1494226]
-
- NCT00117611. Xolair in patients with chronic sinusitis [Effects of anti‐IgE antibody omalizumab (Xolair) on patients with chronic sinusitis and a positive allergen test]. https://clinicaltrials.gov/show/NCT00117611 2005. [CRS: 1770122]
-
- Pinto JM, Mehta N, DiTineo M, Wang J, Baroody FM, Naclerio RM. A randomized, double‐blind, placebo‐controlled trial of anti‐IgE for chronic rhinosinusitis. Rhinology 2010;48(3):318‐24. [CRS: 1569961; PUBMED: 21038023] - PubMed
References to studies excluded from this review
Boguniewicz 2019 {published data only}
-
- Boguniewicz M, Thaci D, Lio PA, Hultsch T, Rossi AB, Eckert L, et al. Dupilumab improves outcomes of concurrent asthma and chronic sino‐nasal conditions in patients with atopic dermatitis‐a pooled analysis of four phase 3 studies (LIBERTY AD SOLO 1 & 2, CHRONOS, and CAFE). Journal of Allergy and Clinical Immunology 2019;143(2 Suppl):AB123. [CRS: 9965833]
Castro 2011 {published data only}
-
- Castro M, Mathur S, Hargreave F, Boulet LP, Xie F, Young J, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo‐controlled study. American Journal of Respiratory and Critical Care Medicine 2011;184(10):1125‐32. [CRS: 1602806; PUBMED: 21852542] - PubMed
-
- Mathur S, Castro M, Hargreave F, Xie F, Wilkins HJ, Henkel T, et al. Efficacy of reslizumab in patients with poorly controlled eosinophilic asthma: subgroup analysis of patients with nasal polyps [Abstract]. Journal of Allergy and Clinical Immunology 2011;127(2 Suppl 1):AB84. [CENTRAL: CN‐00793509]
De Schryver 2015 {published data only}
-
- Schryver E, Zele T, Bachert C, Gevaert P. Comparison of different medical treatment options for CRSwNP: doxycycline, methylprednisolone, mepolizumab and omalizumab. Allergy 2015;70:442. [CENTRAL: CN‐01135998; CRS: 1866506; EMBASE: 72029693]
Gevaert 2006 {published data only}
-
- Gevaert P, Lang‐Loidolt D, Lackner A, Stammberger H, Staudinger H, Zele T, et al. Nasal IL‐5 levels determine the response to anti‐IL‐5 treatment in patients with nasal polyps. Journal of Allergy and Clinical Immunology 2006;118(5):1133‐41. [CENTRAL: CN‐00573474; CRS: 1402690; EMBASE: 2006535588; PUBMED: 17088140] - PubMed
-
- Gevaert P, Zele T, Stammberger H, Staudinger H, Tavernier J, Cauwenberge P, et al. Nasal interleukin‐5 levels determine the response to anti‐interleukin‐5 treatment in nasal polyp patients. 3rd EAACI Davos Meeting in Basic Immunology in Allergy and Clinical Immunology. Davos, Switzerland, 3‐6 February, 2005. 2005. [CENTRAL: CN‐00519575; CRS: 1362295]
-
- Gevaert P, can Zele T, Stammberger H, Sacks H, van Cauwenberge, Bachert C. Anti‐interleukin‐5 treatment in nasal polyposis. Journal of Allergy and Clinical Immunology 2005;115(2):S138. [DOI: 10.1016/j.jaci.2004.12.566] - DOI
Gevaert 2008 {published data only}
-
- Gevaert P, Bruaene N, Blomme K, Sousa AR, Marshal RP, Bachert C. Mepolizumab, a humanised anti‐IL‐5 monoclonal antibody, as treatment of severe nasal polyposis. American Academy of Allergy, Asthma and Immunology (AAAAI) 64th Annual Meeting. Philadelphia, PA, USA, March 14‐18, 2008. Journal of Allergy and Clinical Immunology 2008;121(2 (Suppl 1)):Abstract No. L26. [CRS: 1449627]
Gonzalez‐Diaz 2014 {published data only}
-
- Gonzalez‐Diaz SN, Rangel‐Garza L. Omalizumab efficiency in patients with allergic rhinitis and chronic sinusitis. World Allergy Organization Journal 2014;7(Suppl 1):9. [CRS: 12486358]
Hellings 2017 {published data only}
-
- Hellings P, Bachert C, Mullol J, Hamilos D, Naclerio R, Joish V, et al. Dupilumab improves all ACQ‐5 individual items in patients with chronic rhinosinusitis with nasal polyposis (CRSwNP) and asthma: results from a phase 2a trial. European Respiratory Journal 2017;50(Suppl 61):PA3549. [CRS: 9863239]
-
- Hellings P, Bachert C, Mullol J, Hamilos D, Naclerio R, Mannent L, et al. Dupilumab improves ACQ‐5 items in CRSwNP patients with comorbid asthma. Respiratology 2018;23(Suppl 1):153. [DOI: 10.1111/resp.13268] - DOI
Laidlaw 2019 {published data only}
-
- Laidlaw TM, Mullol J, Fan C, Zhang D, Amin N, Khan A, et al. Dupilumab improves nasal polyp burden and asthma control in patients with CRSwNP and AERD. Journal of Allergy and Clinical Immunology: In Practice 2019;7(7):2462‐5.e1. [CRS: 12486356] - PubMed
Liberty Asthma Quest {published data only}
-
- Bousquet J, Maspero JF, Chipps BE, Corren J, FitzGerald JM, Chen Z, et al. Dupilumab consistently improves rhinoconjunctivitis‐specific health‐related quality of life in patients with uncontrolled, moderate‐to‐severe asthma and comorbid allergic rhinitis: results from the phase 3 LIBERTY ASTHMA QUEST study. Journal of Allergy and Clinical Immunology 2019;143(2 Suppl):AB101. [CENTRAL: CN‐01932317; CRS: 9965843; EMBASE: 2001510554]
-
- Busse W, Maspero JF, Katelaris CH, Saralaya D, Guillonneau S, Zhang B, et al. Dupilumab improves SNOT‐22 scores in asthma patients with chronic rhinosinusitis or nasal polyposis (CRS/NP) in liberty asthma quest. European Respiratory Journal 2018;52(Suppl 62):PA1125. [CRS: 10534564; DOI: 10.1183/13993003.congress-2018.PA1125] - DOI
-
- Busse WW, Maspero JF, Hanania NA, FitzGerald JM, Ford LB, Rice M, et al. Dupilumab improves lung function and reduces severe exacerbation rate in patients with uncontrolled, moderate‐to‐severe asthma with or without comorbid allergic rhinitis: results from the phase 3 LIBERTY ASTHMA QUEST study. Journal of Allergy and Clinical Immunology 2019;143(2 Suppl):AB97. [CENTRAL: CN‐01945651; CRS: 10053593; EMBASE: 2001510335]
-
- Busse WW, Maspero JF, Rabe KF, Papi A, Wenzel SE, Ford LB, et al. Liberty asthma QUEST: phase 3 randomized, double‐blind, placebo‐controlled, parallel‐group study to evaluate dupilumab efficacy/safety in patients with uncontrolled, moderate‐to‐severe asthma. Advances in Therapy 2018;35(5):1‐12. [CENTRAL: CN‐01612071; CRS: 8435206; EMBASE: 622013044; PUBMED: 29725983] - PMC - PubMed
-
- Castro M, Busse WW, Zhang B, Maroni J, Rowe P, Amin N, et al. Dupilumab treatment produces rapid and sustained improvements in FEV1 in patients with uncontrolled, moderate‐to‐severe asthma from the LIBERTY ASTHMA QUEST study. American Journal of Respiratory and Critical Care Medicine 2018;197:A6163. [CENTRAL: CN‐01619354; CRS: 8919994; EMBASE: 622964392]
MUSCA {published data only}
-
- NCT02281318. Efficacy and safety study of mepolizumab adjunctive therapy in participants with severe eosinophilic asthma on markers of asthma control [A randomised, double‐blind, placebo‐controlled, parallel‐group, multi‐centre 24‐week study to evaluate the efficacy and safety of mepolizumab adjunctive therapy in subjects with severe eosinophilic asthma on markers of asthma control]. https://clinicaltrials.gov/show/nct02281318 (first received 3 November 2014). [CRS: 3545753]
-
- Nelsen L, Bradford ES, Bratton DJ, Albers FC, Brusselle G. Improvement in rhinosinusitis health related quality of life in patients with severe eosinophilic asthma. European Respiratory Journal 2017;50(Suppl 61):PA3583. [CRS: 10403903; DOI: 10.1183/1393003.congress-2017.PA3583] - DOI
Naclerio 2017 {published data only}
-
- Naclerio RM, Hamilos DL, Ferguson BJ, Bachert C, Hellings PW, Mullol J, et al. Dupilumab improves sense of smell and reduces anosmia among patients with nasal polyposis and chronic sinusitis: results from a phase 2a trial. Journal of Allergy and Clinical Immunology 2017;139(2 Suppl):AB90. [CRS: 8071505]
NCT00603785 {published data only}
-
- NCT00603785. Effects of anti‐IgE antibody omalizumab on patients with chronic sinusitis [Effects of anti‐IgE antibody omalizumab (Xolair) on patients with chronic sinusitis and a positive allergen test]. https://clinicaltrials.gov/show/nct00603785 (first received 29 January 2008). [CRS: 1643067]
NCT01285323 {published data only}
-
- Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double‐blind, randomised, placebo‐controlled, phase 3 trials. Lancet Respiratory Medicine 2015;3(5):355‐66. [CRS: 1797555; PUBMED: 25736990] - PubMed
-
- NCT01285323. A study to evaluate the efficacy and safety of reslizumab in patients with eosinophilic asthma [A 12‐month, double‐blind, placebo‐controlled, parallel‐group study to evaluate the efficacy and safety of reslizumab (3.0 mg/kg) in the reduction of clinical asthma exacerbations in patients (12‐75 years of age) with eosinophilic asthma]. https://clinicaltrials.gov/show/NCT01285323 (first received 28 January 2011). [CRS: 6716614]
-
- Weinstein SF, Germinaro M, Bardin P, Korn S, Bateman ED. Efficacy of reslizumab with asthma, chronic sinusitis with nasal polyps and elevated blood eosinophils. Journal of Allergy and Clinical Immunology 2016;137(2 Suppl 1):AB86. [CRS: 1865798]
-
- Weinstein SF, Katial RK, Bardin P, Korn S, McDonald M, Garin M, et al. Effects of reslizumab on asthma outcomes in a subgroup of eosinophilic asthma patients with self‐reported chronic rhinosinusitis with nasal polyps. Journal of Allergy & Clinical Immunology in Practice 2019;7(2):589‐96.e3. [CENTRAL: CN‐01922720; CRS: 9618835; EMBASE: 2001152799; PUBMED: 30193936] - PubMed
NCT02170337 {published data only}
-
- NCT02170337. A study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of AMG 282 in healthy subjects and subjects with chronic rhinosinusitis with nasal polyps [A randomized, double‐blind, placebo‐controlled, ascending multiple‐dose study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of AMG 282 in healthy subjects and subjects with chronic rhinosinusitis with nasal polyps]. https://clinicaltrials.gov/show/nct02170337 (first received 19 June 2014). [CRS: 8298441]
NCT02734849 {published data only}
-
- NCT02734849. Study to evaluate multiple doses in patients with nasal polyposis [A phase 2, randomized, double‐blind, placebo‐controlled, study to evaluate multiple doses of AK001 in patients with moderate to severe nasal polyposis]. https://clinicaltrials.gov/show/NCT02734849 (first received 12 April 2016). [CENTRAL: CN‐01415083; CRS: 6977025]
NCT02743871 {published data only}
-
- NCT02743871. Study of PF‐06817024 in healthy subjects, in patients with chronic rhinosinusitis with nasal polyps and in patients with atopic dermatitis [A phase 1, randomized, double‐blind, third‐party open, placebo‐controlled, dose escalating study to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of single and/or multiple intravenous and/or subcutaneous doses of pf‐06817024 in healthy subjects who may BE mildly atopic, subjects with chronic rhinosinusitis with nasal polyps, and subjects with moderate‐severe atopic dermatitis]. https://clinicaltrials.gov/show/NCT02743871 2016. [CRS: 6978629]
Perez De Llano 2018 {published data only}
-
- Perez De Llano L, Meizlik P, McDonald M, Mustafa SS. Reslizumab decreases nasal adverse events and upper respiratory‐associated concomitant medication use in patients with eosinophilic asthma and nasal polyps. Allergy 2018;73(Suppl 105):91‐2. [CRS: 12486354]
Tajiri 2013 {published data only}
-
- Tajiri T, Matsumoto H, Hiraumi H, Ikeda H, Morita K, Izuhara K, et al. Efficacy of omalizumab in eosinophilic chronic rhinosinusitis patients with asthma. Annals of Allergy, Asthma, & Immunology 2013;110(5):387‐8. [CRS: 12486360] - PubMed
Zangrilli 2019 {published data only}
-
- Zangrilli JG, Maspero J, Harrison T, Werkstrom V, Wu Y. Clinical efficacy of benralizumab in patients with severe, uncontrolled eosinophilic asthma and nasal polyposis: pooled Analysis of the SIROCCO and CALIMA Trials. Pneumologie (Stuttgart, Germany) 2019;73(Suppl 1):AB12. [CRS: 11766888; DOI: 10.1016/j.jaci.2017.12.038] - DOI
References to ongoing studies
NCT02772419 {published data only}
-
- NCT02772419. Study of benralizumab (KHK4563) in patients with eosinophilic chronic rhinosinusitis [A phase 2, double‐blind, placebo‐controlled study of benralizumab (KHK4563) in patients with eosinophilic chronic rhinosinusitis]. https://clinicaltrials.gov/show/NCT02772419 (first received 13 May 2016). [CENTRAL: CN‐01415166; CRS: 6978699]
NCT02799446 {published data only}
-
- NCT02799446. Effect of reslizumab in chronic rhinosinusitis [Efficacy of reslizumab for the treatment of chronic rhinosinusitis a double blind, randomized, placebo‐controlled, phase III trial]. https://clinicaltrials.gov/show/NCT02799446 (first received 14 June 2016). [CRS: 6978626]
NCT03450083 {published data only}
-
- NCT03450083. Benralizumab effect on severe chronic rhinosinusitis with eosinophilic polyposis [Benralizumab effect on severe chronic rhinosinusitis with eosinophilic polyposis: a phase II randomized placebo controlled trial]. https://clinicaltrials.gov/show/nct03450083 (first received 17 August 2017). [CRS: 8239610]
NCT03614923 {published data only}
-
- NCT03614923. Etokimab in adult patients with chronic rhinosinusitis with nasal polyps (CRSwNP) [A phase 2 double‐blind, placebo‐controlled multi‐dose study to investigate etokimab (ANB020) activity in adult patients with chronic rhinosinusitis with nasal polyps]. https://clinicaltrials.gov/show/NCT03614923 (first received 3 August 2018). [CRS: 9157986]
OSTRO {published data only}
-
- NCT03401229. Efficacy and safety study of benralizumab for patients with severe nasal polyposis [A multicenter, randomized, double‐blind, parallel‐group, placebo‐controlled phase 3 efficacy and safety study of benralizumab in patients with severe nasal polyposis]. https://clinicaltrials.gov/show/NCT03401229 (first received 17 January 2018). [CRS: 8275488]
POLYP 1 {unpublished data only}
-
- NCT03280550. A clinical trial of omalizumab in participants with chronic rhinosinusitis with nasal polyps [A phase III, randomized, multicenter, double‐blind, placebo‐controlled clinical trial of omalizumab in patients with chronic rhinosinusitis with nasal polyps]. https://clinicaltrials.gov/show/NCT03280550 (first received 12 September 2017). [CENTRAL: CN‐01415214; CRS: 6978602]
POLYP 2 {unpublished data only}
-
- NCT03280537. A clinical trial of omalizumab in participants with chronic rhinosinusitis with nasal polyps [A phase III, randomized, multicenter, double‐blind, placebo‐controlled clinical trial of omalizumab in patients with chronic rhinosinusitis with nasal polyps]. https://clinicaltrials.gov/show/NCT03280537 (first received 12 September 2017). [CENTRAL: CN‐01415096; CRS: 6977504]
SYNAPSE {published data only}
-
- EUCTR2016‐004255‐70‐SE. Effect of mepolizumab in severe bilateral nasal polyps [A randomised, double‐blind, parallel group PhIII study to assess the clinical efficacy and safety of 100 mg SC mepolizumab as an add on to maintenance treatment in adults with severe bilateral nasal polyps ‐ SYNAPSE (StudY in NAsal Polyps patients to assess the Safety and Efficacy of mepolizumab)]. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2016‐004255‐70/SE (first received 4 October 2017). [CRS: 10787349]
-
- NCT03085797. Effect of mepolizumab in severe bilateral nasal polyps [A randomised, double‐blind, parallel group PhIII study to assess the clinical efficacy and safety of 100 mg SC mepolizumab AS an add on to maintenance treatment in adults with severe bilateral nasal polyps ‐ SYNAPSE (study in nasal polyps patients to assess the safety and efficacy of mepolizumab)]. https://clinicaltrials.gov/show/NCT03085797 (first received 21 March 2017). [CRS: 6978655]
Additional references
Cho 2012
Chong 2016a
Chong 2016b
Chong 2016c
DeMarcantonio 2011
-
- DeMarcantonio MA, Han JK. Nasal polyps: pathogenesis and treatment implications. Otolaryngologic Clinics of North America 2011;44(3):685‐95, ix. - PubMed
Ebbens 2010
-
- Ebbens FA, Toppila‐Salmi SK, Renkonen JA, Renkonen RL, Mullol J, Drunen CM, et al. Endothelial L‐selectin ligand expression in nasal polyps. Allergy 2010;65(1):95‐102. - PubMed
Ebbens 2011
-
- Ebbens FA, Toppila‐Salmi S, Groot EJ, Renkonen J, Renkonen R, Drunen CM, et al. Predictors of post‐operative response to treatment: a double blind placebo controlled study in chronic rhinosinusitis patients. Rhinology 2011;49(4):413‐9. - PubMed
Egger 1997
EPOS 2007
-
- Fokkens W, Lund V, Mullol J, European Position Paper on Rhinosinusitis and Nasal Polyps Group. European position paper on rhinosinusitis and nasal polyps 2007. Rhinology. Supplement 2007;45 Suppl 20:1‐136. - PubMed
EPOS 2012
-
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinology. Supplement 2012;50 Suppl 23:1‐298. - PubMed
FDA 2018
-
- US Food, Drug Administration. CFR ‐ Code of Federal Regulations Title 21. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?f... (accessed 18 February 2019).
Gliklich 1995
-
- Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngology ‐ Head and Neck Surgery 1995;113(1):104‐9. - PubMed
Handbook 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Handbook 2019
-
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hastan 2011
-
- Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, et al. Chronic rhinosinusitis in Europe ‐ an underestimated disease. A GA2LEN study. Allergy 2011;66(9):1216‐23. - PubMed
Head 2016a
Head 2016b
Head 2016c
Head 2018
Hoehle 2019
-
- Hoehle LP, Phillips KM, Speth MM, Caradonna DS, Gray ST, Sedaghat AR. Responsiveness and minimal clinically important difference for the EQ‐5D in chronic rhinosinusitis. Rhinology 2019;57(2):110‐6. - PubMed
Hong 2015
Hopkins 2009
Hopkins 2018
-
- Hopkins C, Hettige R, Soni‐Jaiswal A, Lakhani R, Carrie S, et al. CHronic Rhinosinusitis Outcome MEasures (CHROME), developing a core outcome set for trials of interventions in chronic rhinosinusitis. Rhinology 2018;56(1):22‐32. [PUBMED: 29306959] - PubMed
Jefferson 2018
Kariyawasam 2019
Kern 2008
Keswani 2012
Larsen 2004
-
- Larsen P, Tos M. Origin of nasal polyps: an endoscopic autopsy study. Laryngoscope 2004;114(4):710‐9. - PubMed
Marshal 2018
McDonald 2017
-
- McDonald S, Noel‐Storr AH, Thomas J. Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane reviews. Global Evidence Summit; 2017 Sep 13‐17; Cape Town, South Africa. 2017.
NICE 2019
-
- National Institute for Health and Care Excellence (NICE). Dupilumab for treating chronic rhinosinusitis with nasal polyps ID1179. https://www.nice.org.uk/guidance/proposed/gid‐ta10450 (accessed 18 February 2019).
Noel‐Storr 2018
-
- Noel‐Storr AH, The Project Transform Team. Cochrane Crowd: new ways of working together to produce health evidence. Evidence Live; 2018 Jun 18‐20; Oxford, UK. 2018.
Philpott 2015
Ragab 2004
-
- Ragab SM, Lund VJ, Scadding G. Evaluation of the medical and surgical treatment of chronic rhinosinusitis: a prospective, randomised, controlled trial. Laryngoscope 2004;114(5):923‐30. - PubMed
Ragab 2010
-
- Ragab SM, Lund VJ, Scadding G, Saleh HA, Khalifa MA. Impact of chronic rhinosinusitis therapy on quality of life: a prospective randomized controlled trial. Rhinology 2010;48(3):305‐11. - PubMed
RevMan Web 2019 [Computer program]
-
- The Cochrane Collaboration. Review Manager Web (RevMan Web). The Cochrane Collaboration, 2019.
Rivero 2017
Simmonds 2017
Smith 2018
Smith 2019
Sterne 2019
Tan 2011
Thomas 2017
Tomassen 2011
-
- Tomassen P, Zele T, Zhang N, Perez‐Novo C, Bruaene N, Gevaert P, et al. Pathophysiology of chronic rhinosinusitis. Proceedings of the American Thoracic Society 2011;8(1):115‐20. - PubMed
Tsetsos 2018
van Drunen 2009
-
- Drunen CM, Reinartz SM, Wigman J, Fokkens W. Inflammation in chronic rhinosinusitis and nasal polyposis. Immunology and Allergy Clinics of North America 2009;29(4):621‐9. - PubMed
Wallace 2017
-
- Wallace BC, Noel‐Storr AH, Marshall IJ, Cohen AM, Smalheiser NR, et al. Identifying reports of randomized controlled trials (RCTs) via a hybrid machine learning and crowdsourcing approach. Journal of the American Medical Informatics Association 2017;24(6):1165‐8. [DOI: 10.1093/jamia/ocx053] - DOI - PMC - PubMed
Zhang 2008
-
- Zhang N, Zele T, Perez‐Novo C, Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T‐effector cells orchestrate mucosal inflammation in chronic sinus disease. Journal of Allergy and Clinical Immunology 2008;122(5):961‐8. - PubMed
Zhang 2009
-
- Zhang XH, Lu X, Long XB, You XJ, Gao QX, Cui YH, et al. Chronic rhinosinusitis with and without nasal polyps is associated with decreased expression of glucocorticoid‐induced leucine zipper. Clinical and Experimental Allergy 2009;39(5):647‐54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous