G-Protein-Coupled Receptors in CNS: A Potential Therapeutic Target for Intervention in Neurodegenerative Disorders and Associated Cognitive Deficits
- PMID: 32102186
- PMCID: PMC7072884
- DOI: 10.3390/cells9020506
G-Protein-Coupled Receptors in CNS: A Potential Therapeutic Target for Intervention in Neurodegenerative Disorders and Associated Cognitive Deficits
Abstract
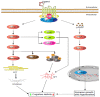
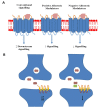
Neurodegenerative diseases are a large group of neurological disorders with diverse etiological and pathological phenomena. However, current therapeutics rely mostly on symptomatic relief while failing to target the underlying disease pathobiology. G-protein-coupled receptors (GPCRs) are one of the most frequently targeted receptors for developing novel therapeutics for central nervous system (CNS) disorders. Many currently available antipsychotic therapeutics also act as either antagonists or agonists of different GPCRs. Therefore, GPCR-based drug development is spreading widely to regulate neurodegeneration and associated cognitive deficits through the modulation of canonical and noncanonical signals. Here, GPCRs' role in the pathophysiology of different neurodegenerative disease progressions and cognitive deficits has been highlighted, and an emphasis has been placed on the current pharmacological developments with GPCRs to provide an insight into a potential therapeutic target in the treatment of neurodegeneration.
Keywords: cannabinoid receptor; metabotropic glutamate receptor; orphan G-protein-coupled receptors; serotonin; seven transmembranes.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures
References
-
- Jakaria M., Azam S., Cho D.-Y., Haque M.E., Kim I.-S., Choi D.-K. The Methanol Extract of Allium cepa L. Protects Inflammatory Markers in LPS-Induced BV-2 Microglial Cells and Upregulates the Antiapoptotic Gene and Antioxidant Enzymes in N27-A Cells. Antioxidants. 2019;8:348. doi: 10.3390/antiox8090348. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

