Direct Base-Assisted C‒H Cyclonickelation of 6-Phenyl-2,2'-bipyridine
- PMID: 32102281
- PMCID: PMC7070369
- DOI: 10.3390/molecules25040997
Direct Base-Assisted C‒H Cyclonickelation of 6-Phenyl-2,2'-bipyridine
Abstract
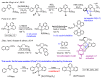
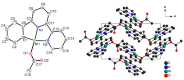
The organonickel complexes [Ni(Phbpy)X] (X = Br, OAc, CN) were obtained for the first time in a direct base-assisted arene C(sp2)-H cyclometalation reaction from the rather unreactive precursor materials NiX2 and HPhbpy (6-phenyl-2,2'-bipyridine) or from the versatile precursor [Ni(HPhbpy)Br2]2. Different from previously necessary C‒Br oxidative addition at Ni(0), an extended scan of reaction conditions allowed quantitative access to the title compound from Ni(II) on synthetically useful timescales through base-assisted C‒H activation in nonpolar media at elevated temperature. Optimisation of the reaction conditions (various bases, solvents, methods) identified 1:2 mixtures of acetate and carbonate as unrivalled synergetic base pairs in the optimum protocol that holds promise as a readily usable and easily tuneable access to a wide range of direct nickelation products. While for the base-assisted C‒H metalation of the noble metals Ru, Ir, Rh, or Pd, this acetate/carbonate method has been established for a few years, our study represents the leap into the world of the base metals of the 3d series.
Keywords: C–H activation; base-assisted; cyclometalation; cyclonickelation; organonickel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Cope A.C., Siekman R.W. Formation of Covalent Bonds from Platinum or Palladium to Carbon by Direct Substitution. J. Am. Chem. Soc. 1965;87:3272–3273. doi: 10.1021/ja01092a063. - DOI
-
- Cope A.C., Friedrich E.C. Electrophilic Aromatic Substitution Reactions by Platinum(II) and Palladium(II) Chlorides on N,N-Dimethylbenzylamines. J. Am. Chem. Soc. 1968;90:909–913. doi: 10.1021/ja01006a012. - DOI
-
- Chassot L., Mueller E., Von Zelewsky A. cis-Bis(2-phenylpyridine)platinum(II) (CBPPP): A Simple Molecular Platinum Compound. Inorg. Chem. 1984;23:4249–4253. doi: 10.1021/ic00193a030. - DOI
-
- Constable E.C., Henney R.P.G., Leese T.A., Tocher D.A. Cyclometallation Reactions of 6-Phenyl-2,2’-bipyridine; a Potential C,N,N-Donor Analogue of 2,2’:6’,2”-Terpyridine. Crystal and Molecular Structure of Dichlorobis(6-phenyl-2,2’-bipyridine)ruthenium(II) J. Chem. Soc., Dalton Trans. 1990:443–449. doi: 10.1039/DT9900000443. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

