(R)-Salbutamol Improves Imiquimod-Induced Psoriasis-Like Skin Dermatitis by Regulating the Th17/Tregs Balance and Glycerophospholipid Metabolism
- PMID: 32102363
- PMCID: PMC7072797
- DOI: 10.3390/cells9020511
(R)-Salbutamol Improves Imiquimod-Induced Psoriasis-Like Skin Dermatitis by Regulating the Th17/Tregs Balance and Glycerophospholipid Metabolism
Abstract
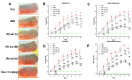
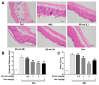
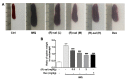
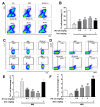
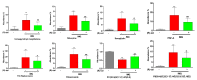
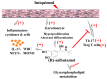
Psoriasis is a skin disease that is characterized by a high degree of inflammation caused by immune dysfunction. (R)-salbutamol is a bronchodilator for asthma and was reported to alleviate immune system reactions in several diseases. In this study, using imiquimod (IMQ)-induced mouse psoriasis-like dermatitis model, we evaluated the therapeutic effects of (R)-salbutamol in psoriasis in vivo, and explored the metabolic pathway involved. The results showed that, compared with IMQ group, (R)-salbutamol treatment significantly ameliorated psoriasis, reversed the suppressive effects of IMQ on differentiation, extreme keratinocyte proliferation, and infiltration of inflammatory cells. Enzyme-linked immunosorbent assays (ELISA) showed that (R)-salbutamol markedly reduced the plasma levels of IL-17. Cell analysis using flow cytometry showed that (R)-salbutamol decreased the proportion of CD4+ Th17+ T cells (Th17), whereas it increased the percentage of CD25+ Foxp3+ regulatory T cells (Tregs) in the spleens. (R)-salbutamol also decreased the weight ratio of spleen to body. Furthermore, untargeted metabolomics showed that (R)-salbutamol affected three metabolic pathways, including (i) arachidonic acid metabolism, (ii) sphingolipid metabolism, and (iii) glycerophospholipid metabolism. These results demonstrated that (R)-salbutamol can alleviate IMQ-induced psoriasis through regulating Th17/Tregs cell response and glycerophospholipid metabolism. It may provide a new use of (R)-salbutamol in the management of psoriasis.
Keywords: (R)-salbutamol; Th17/Tregs; immune-regulation; metabolomics; psoriasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

