Reactive & Efficient: Organic Azides as Cross-Linkers in Material Sciences
- PMID: 32102403
- PMCID: PMC7070646
- DOI: 10.3390/molecules25041009
Reactive & Efficient: Organic Azides as Cross-Linkers in Material Sciences
Abstract
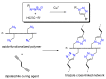
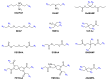
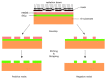
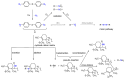
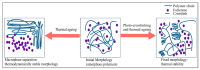
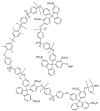
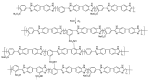
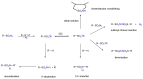
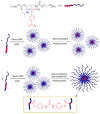
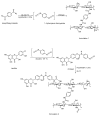
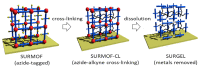
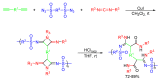
The exceptional reactivity of the azide group makes organic azides a highly versatile family of compounds in chemistry and the material sciences. One of the most prominent reactions employing organic azides is the regioselective copper(I)-catalyzed Huisgen 1,3-dipolar cycloaddition with alkynes yielding 1,2,3-triazoles. Other named reactions include the Staudinger reduction, the aza-Wittig reaction, and the Curtius rearrangement. The popularity of organic azides in material sciences is mostly based on their propensity to release nitrogen by thermal activation or photolysis. On the one hand, this scission reaction is accompanied with a considerable output of energy, making them interesting as highly energetic materials. On the other hand, it produces highly reactive nitrenes that show extraordinary efficiency in polymer crosslinking, a process used to alter the physical properties of polymers and to boost efficiencies of polymer-based devices such as membrane fuel cells, organic solar cells (OSCs), light-emitting diodes (LEDs), and organic field-effect transistors (OFETs). Thermosets are also suitable application areas. In most cases, organic azides with multiple azide functions are employed which can either be small molecules or oligo- and polymers. This review focuses on nitrene-based applications of multivalent organic azides in the material and life sciences.
Keywords: azides; nitrenes; photochemistry; polymers; thermosets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


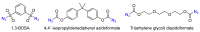
References
-
- Griess P. On a new series of bodies in which nitrogen is substituted for hydrogen. Philos. Trans. R. Soc. Lond. 1864;154:667–731. doi: 10.1039/JS8651800268. - DOI
-
- Hepher M., Wagner H.M. Azide Resin Photolithographic Composition. No. US2852379A. US Patent. 1958 Sep 16;
-
- Manchado M.A.L., Kenny J.M. Use of benzene-1,3-bis(sulfonyl)azide as crosslinking agent of TPVS based on EPDM rubber-polyolefin blends. Rubber Chem. Technol. 2001;74:198–210. doi: 10.5254/1.3544944. - DOI
-
- Baker D.A., East G.C., Mukhopadhyay S.K. Mechanical and thermal properties of acrylic fibers crosslinked with disulfonyl azides. J. Appl. Polym. Sci. 2002;84:1309–1319. doi: 10.1002/app.2349. - DOI
-
- Avadanei M., Grigoriu G.E., Barboiu V. Photocrosslinking of poly-1,2-butadiene in the presence of benzophenone and aromatic diazide. Rev. Roum. De Chim. 2003;48:813–819.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

