Microfluidic Device for On-Chip Immunophenotyping and Cytogenetic Analysis of Rare Biological Cells
- PMID: 32102446
- PMCID: PMC7072755
- DOI: 10.3390/cells9020519
Microfluidic Device for On-Chip Immunophenotyping and Cytogenetic Analysis of Rare Biological Cells
Abstract
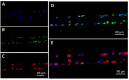
The role of circulating plasma cells (CPCs) and circulating leukemic cells (CLCs) as biomarkers for several blood cancers, such as multiple myeloma and leukemia, respectively, have recently been reported. These markers can be attractive due to the minimally invasive nature of their acquisition through a blood draw (i.e., liquid biopsy), negating the need for painful bone marrow biopsies. CPCs or CLCs can be used for cellular/molecular analyses as well, such as immunophenotyping or fluorescence in situ hybridization (FISH). FISH, which is typically carried out on slides involving complex workflows, becomes problematic when operating on CLCs or CPCs due to their relatively modest numbers. Here, we present a microfluidic device for characterizing CPCs and CLCs using immunofluorescence or FISH that have been enriched from peripheral blood using a different microfluidic device. The microfluidic possessed an array of cross-channels (2-4 µm in depth and width) that interconnected a series of input and output fluidic channels. Placing a cover plate over the device formed microtraps, the size of which was defined by the width and depth of the cross-channels. This microfluidic chip allowed for automation of immunofluorescence and FISH, requiring the use of small volumes of reagents, such as antibodies and probes, as compared to slide-based immunophenotyping and FISH. In addition, the device could secure FISH results in <4 h compared to 2-3 days for conventional FISH.
Keywords: circulating leukemia cells; circulating plasma cells; fish; immunophenotyping; liquid biopsy; microfluidics.
Conflict of interest statement
S.A.S. and M.L.H. hold equity shares in BioFluidica, Inc., a company that holds commercialization rights to the cell isolation technology described herein. M.A.W declares COI as a spouse of a BioFluidica, Inc. employee.
Figures






References
-
- Macías M., Alegre E., Díaz-Lagares A., Patiño-García A., Pérez-Gracia J.L., Sanmamed M.F., López-López R., Varo N., Gonzalez A. Advances in Virus Research. Elsevier BV; Bethesda, MD, USA: 2018. Liquid Biopsy: From Basic Research to Clinical Practice; pp. 73–119. - PubMed
-
- Jackson J.M., Taylor J.B., Witek M.A., Hunsucker S., Waugh J.P., Fedoriw Y., Shea T.C., Soper S.A., Armistead P.M. Microfluidics for the detection of minimal residual disease in acute myeloid leukemia patients using circulating leukemic cells selected from blood. Analyst. 2016;141:640–651. doi: 10.1039/C5AN01836F. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

