A Mortality Prediction Rule for Hematology Patients with Invasive Aspergillosis Based on Serum Galactomannan Kinetics
- PMID: 32102465
- PMCID: PMC7073547
- DOI: 10.3390/jcm9020610
A Mortality Prediction Rule for Hematology Patients with Invasive Aspergillosis Based on Serum Galactomannan Kinetics
Abstract
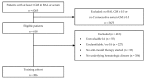
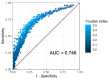
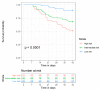
In invasive aspergillosis (IA), an early and adequate assessment of the response to the initial antifungal therapy remains problematic. We retrospectively analyzed 206 hematology patients with proven or probable IA, and collected serial serum galactomannan (sGM) values and survival status through week 6 and week 12. We created a model for survival at week 6 based on the sGM taken at baseline and on early sGM kinetics. This resulted in a rule predicting that patients with a baseline sGM index >1.4, who failed to lower that index to <0.5 after one week, had a mortality rate of 48.1% at week 6. Conversely, patients presenting with a baseline sGM index ≤1.4 that obtained a negative sGM (<0.5) after one week, had a mortality that was almost five times lower at only 10.1% by week 6. These findings were confirmed in an external cohort from an independent prospective study. In conclusion, sGM kinetics correlate well with treatment outcomes in hematology patients with IA. We present a rule which is easy to use at the bedside and has good accuracy in predicting week 6 survival.
Keywords: galactomannan; invasive aspergillosis; kinetics; mortality; outcome; prognosis.
Conflict of interest statement
T.M. received an unrestricted research grant from Gilead Sciences; served as a consultant for Gilead Sciences, Pfizer, and Celgene; and received non-financial support from MSD/Merck, Gilead Sciences, Pfizer, OLM Diagnostics, IMMY, and FUJIFILM Wako. K.L. received consultancy fees from MSD, SMB Laboratoires Brussels, and Gilead; travel support from Pfizer and MSD; and speaker fees from Gilead, MSD, FUJIFILM WAKO and Pfizer. J.M. received research grants from Merck/MSD, Gilead Sciences and Pfizer; is a consultant to Astellas, Basilea, Bio‐Rad, Merck/MSD, Pfizer, Schering‐Plough, F2G, Gilead Sciences, Cidara, Scynexis, Amplyx and Luminex; and served on the speaker's bureau of Astellas, Gilead Sciences, Bio‐Rad, Merck/MSD, Pfizer, F2G, Schering‐Plough, Basilea and Shire/Takeda. L.C. has received grant support and has been advisor/consultant for Pfizer, Gilead, Astellas and Merck Sharp and Dohme (MSD). J.W. has no conflicts to report.
Figures

References
-
- Hope W.W., Petraitis V., Petraitiene R., Aghamolla T., Bacher J., Walsh T.J. The Initial 96 Hours of Invasive Pulmonary Aspergillosis: Histopathology, Comparative Kinetics of Galactomannan and (1→3)-β-d-Glucan, and Consequences of Delayed Antifungal Therapy. Antimicrob. Agents Chemother. 2010;54:4879–4886. doi: 10.1128/AAC.00673-10. - DOI - PMC - PubMed
-
- de Pauw B., Walsh T.J., Donnelly J.P., Stevens D.A., Edwards J.E., Calandra T., Pappas P.G., Maertens J., Lortholary O., Kauffman C.A., et al. Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008;46:1813–1821. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

