The Hypoxic Proteome and Metabolome of Barley (Hordeum vulgare L.) with and without Phytoglobin Priming
- PMID: 32102473
- PMCID: PMC7073221
- DOI: 10.3390/ijms21041546
The Hypoxic Proteome and Metabolome of Barley (Hordeum vulgare L.) with and without Phytoglobin Priming
Abstract
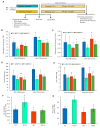
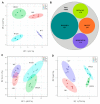
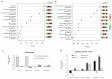
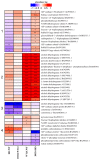
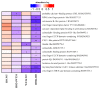
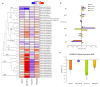
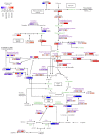
Overexpression of phytoglobins (formerly plant hemoglobins) increases the survival rate of plant tissues under hypoxia stress by the following two known mechanisms: (1) scavenging of nitric oxide (NO) in the phytoglobin/NO cycle and (2) mimicking ethylene priming to hypoxia when NO scavenging activates transcription factors that are regulated by levels of NO and O2 in the N-end rule pathway. To map the cellular and metabolic effects of hypoxia in barley (Hordeum vulgare L., cv. Golden Promise), with or without priming to hypoxia, we studied the proteome and metabolome of wild type (WT) and hemoglobin overexpressing (HO) plants in normoxia and after 24 h hypoxia (WT24, HO24). The WT plants were more susceptible to hypoxia than HO plants. The chlorophyll a + b content was lowered by 50% and biomass by 30% in WT24 compared to WT, while HO plants were unaffected. We observed an increase in ROS production during hypoxia treatment in WT seedlings that was not observed in HO seedlings. We identified and quantified 9694 proteins out of which 1107 changed significantly in abundance. Many proteins, such as ion transporters, Ca2+-signal transduction, and proteins related to protein degradation were downregulated in HO plants during hypoxia, but not in WT plants. Changes in the levels of histones indicates that chromatin restructuring plays a role in the priming of hypoxia. We also identified and quantified 1470 metabolites, of which the abundance of >500 changed significantly. In summary the data confirm known mechanisms of hypoxia priming by ethylene priming and N-end rule activation; however, the data also indicate the existence of other mechanisms for hypoxia priming in plants.
Keywords: N-end rule; anaerobiosis; ethylene; hemoglobin; histones; stress priming.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- CRED/UNISDR . The Human Cost of Weather Related Disasters, 1995–2015. CRED; Brussels, Belgium: 2015. pp. 5–16.
-
- WFP World Food Programme Ready to Respond to Food Crisis in Malawi. [(accessed on 10 February 2020)]; Available online: https://www.wfp.org/news/news-release/world-food-programme-ready-respond....
-
- Sasidharan R., Bailey-Serres J., Ashikari M., Atwell B.J., Colmer T.D., Fagerstedt K., Fukao T., Geigenberger P., Hebelstrup K.H., Hill R.D., et al. Community recommendations on terminology and procedures used in flooding and low oxygen stress research. New Phytol. 2017;214:1403–1407. doi: 10.1111/nph.14519. - DOI - PubMed
-
- Yordanova R.Y., Popova L.P. Flooding-induced changes in photosynthesis and oxidative status in maize plants. Acta Physiol. Plant. 2007;29:535–541. doi: 10.1007/s11738-007-0064-z. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

