Remodeling of Intracellular Ca2+ Homeostasis in Rat Hippocampal Neurons Aged In Vitro
- PMID: 32102482
- PMCID: PMC7073228
- DOI: 10.3390/ijms21041549
Remodeling of Intracellular Ca2+ Homeostasis in Rat Hippocampal Neurons Aged In Vitro
Abstract
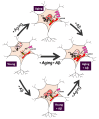
Aging is often associated with a cognitive decline and a susceptibility to neuronal damage. It is also the most important risk factor for neurodegenerative disorders, particularly Alzheimer's disease (AD). AD is related to an excess of neurotoxic oligomers of amyloid β peptide (Aβo); however, the molecular mechanisms are still highly controversial. Intracellular Ca2+ homeostasis plays an important role in the control of neuronal activity, including neurotransmitter release, synaptic plasticity, and memory storage, as well as neuron cell death. Recent evidence indicates that long-term cultures of rat hippocampal neurons, resembling aged neurons, undergo cell death after treatment with Aβo, whereas short-term cultures, resembling young neurons, do not. These in vitro changes are associated with the remodeling of intracellular Ca2+ homeostasis with aging, thus providing a simplistic model for investigating Ca2+ remodeling in aging. In vitro aged neurons show increased resting cytosolic Ca2+ concentration, enhanced Ca2+ store content, and Ca2+ release from the endoplasmic reticulum (ER). Ca2+ transfer from the endoplasmic reticulum (ER) to mitochondria is also enhanced. Aged neurons also show decreased store-operated Ca2+ entry (SOCE), a Ca2+ entry pathway related to memory storage. At the molecular level, in vitro remodeling is associated with changes in the expression of Ca2+ channels resembling in vivo aging, including changes in N-methyl-D-aspartate NMDA receptor and inositol 1,4,5-trisphosphate (IP3) receptor isoforms, increased expression of the mitochondrial calcium uniporter (MCU), and decreased expression of Orai1/Stim1, the molecular players involved in SOCE. Additionally, Aβo treatment exacerbates most of the changes observed in aged neurons and enhances susceptibility to cell death. Conversely, the solely effect of Aβo in young neurons is to increase ER-mitochondria colocalization and enhance Ca2+ transfer from ER to mitochondria without inducing neuronal damage. We propose that cultured rat hippocampal neurons may be a useful model to investigate Ca2+ remodeling in aging and in age-related neurodegenerative disorders.
Keywords: Alzheimer’s disease; aging; amyloid beta oligomers; calcium; endoplasmic reticulum; hippocampal neurons; mitochondria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Go A.S., Mozaffarian D., Roger V.L., Benjamin E.J., Berry J.D., Borden W.B., Bravata D.M., Dai S., Ford E.S., Fox C.S., et al. Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

