Platelets Disseminate Extracellular Vesicles in Lymph in Rheumatoid Arthritis
- PMID: 32102567
- PMCID: PMC8073225
- DOI: 10.1161/ATVBAHA.119.313698
Platelets Disseminate Extracellular Vesicles in Lymph in Rheumatoid Arthritis
Abstract
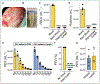
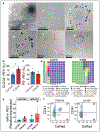
Objective: The lymphatic system is a circulatory system that unidirectionally drains the interstitial tissue fluid back to blood circulation. Although lymph is utilized by leukocytes for immune surveillance, it remains inaccessible to platelets and erythrocytes. Activated cells release submicron extracellular vesicles (EV) that transport molecules from the donor cell. In rheumatoid arthritis, EV accumulate in the joint where they can interact with numerous cellular lineages. However, whether EV can exit the inflamed tissue to recirculate is unknown. Here, we investigated whether vascular leakage that occurs during inflammation could favor EV access to the lymphatic system. Approach and Results: Using an in vivo model of autoimmune inflammatory arthritis, we show that there is an influx of platelet EV, but not EV from erythrocytes or leukocytes, in joint-draining lymph. In contrast to blood platelet EV, lymph platelet EV lacked mitochondrial organelles and failed to promote coagulation. Platelet EV influx in lymph was consistent with joint vascular leakage and implicated the fibrinogen receptor α2bβ3 and platelet-derived serotonin.
Conclusions: These findings show that platelets can disseminate their EV in fluid that is inaccessible to platelets and beyond the joint in this disease.
Keywords: arthritis; extracellular vesicles; inflammation; leukocytes; lymphatic system; platelets.
Figures
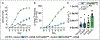

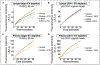

References
-
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. The Lancet. 2016;6736:1–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

