Acute Nicotine Exposure Alters Ventral Tegmental Area Inhibitory Transmission and Promotes Diazepam Consumption
- PMID: 32102779
- PMCID: PMC7082131
- DOI: 10.1523/ENEURO.0348-19.2020
Acute Nicotine Exposure Alters Ventral Tegmental Area Inhibitory Transmission and Promotes Diazepam Consumption
Abstract
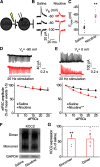
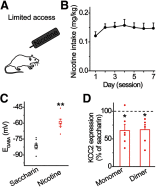
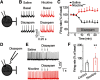
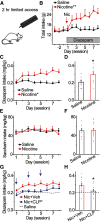
Nicotine use increases the risk for subsequent abuse of other addictive drugs, but the biological basis underlying this risk remains largely unknown. Interactions between nicotine and other drugs of abuse may arise from nicotine-induced neural adaptations in the mesolimbic dopamine (DA) system, a common pathway for the reinforcing effects of many addictive substances. Previous work identified nicotine-induced neuroadaptations that alter inhibitory transmission in the ventral tegmental area (VTA). Here, we test whether nicotine-induced dysregulation of GABAergic signaling within the VTA increases the vulnerability for benzodiazepine abuse that has been reported in smokers. We demonstrate in rats that nicotine exposure dysregulates diazepam-induced inhibition of VTA GABA neurons and increases diazepam consumption. In VTA GABA neurons, nicotine impaired KCC2-mediated chloride extrusion, depolarized the GABAA reversal potential, and shifted the pharmacological effect of diazepam on GABA neurons from inhibition toward excitation. In parallel, nicotine-related alterations in GABA signaling observed ex vivo were associated with enhanced diazepam-induced inhibition of lateral VTA DA neurons in vivo Targeting KCC2 with the agonist CLP290 normalized diazepam-induced effects on VTA GABA transmission and reduced diazepam consumption following nicotine administration to the control level. Together, our results provide insights into midbrain circuit alterations resulting from nicotine exposure that contribute to the abuse of other drugs, such as benzodiazepines.
Keywords: GABA; KCC2; benzodiazepines; chloride; dopamine; mesolimbic.
Copyright © 2020 Ostroumov et al.
Figures



References
-
- Büchel C, Peters J, Banaschewski T, Bokde AL, Bromberg U, Conrod PJ, Flor H, Papadopoulos D, Garavan H, Gowland P, Heinz A, Walter H, Ittermann B, Mann K, Martinot JL, Paillère-Martinot ML, Nees F, Paus T, Pausova Z, Poustka L, Rietschel M, et al. (2017) Blunted ventral striatal responses to anticipated rewards foreshadow problematic drug use in novelty-seeking adolescents. Nat Commun 8:14140. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
