Cost effectiveness of an intervention to increase uptake of hepatitis C virus testing and treatment (HepCATT): cluster randomised controlled trial in primary care
- PMID: 32102782
- PMCID: PMC7190058
- DOI: 10.1136/bmj.m322
Cost effectiveness of an intervention to increase uptake of hepatitis C virus testing and treatment (HepCATT): cluster randomised controlled trial in primary care
Erratum in
-
Cost effectiveness of an intervention to increase uptake of hepatitis C virus testing and treatment (HepCATT): cluster randomised controlled trial in primary care.BMJ. 2021 May 5;373:n1130. doi: 10.1136/bmj.n1130. BMJ. 2021. PMID: 33952529 Free PMC article. No abstract available.
Abstract
Objective: To evaluate the effectiveness and cost effectiveness of a complex intervention in primary care that aims to increase uptake of hepatitis C virus (HCV) case finding and treatment.
Design: Pragmatic, two armed, practice level, cluster randomised controlled trial and economic evaluation.
Setting and participants: 45 general practices in South West England (22 randomised to intervention and 23 to control arm). Outcome data were collected from all intervention practices and 21/23 control practices. Total number of flagged patients was 24 473 (about 5% of practice list).
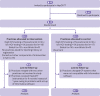
Intervention: Electronic algorithm and flag on practice systems identifying patients with HCV risk markers (such as history of opioid dependence or HCV tests with no evidence of referral to hepatology), staff educational training in HCV, and practice posters/leaflets to increase patients' awareness. Flagged patients were invited by letter for an HCV test (with one follow-up) and had on-screen pop-ups to encourage opportunistic testing. The intervention lasted one year, with practices recruited April to December 2016.
Main outcome measures: Primary outcome: uptake of HCV testing.
Secondary outcomes: number of positive HCV tests and yield (proportion HCV positive); HCV treatment assessment at hepatology; cost effectiveness.
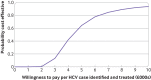
Results: Baseline HCV testing of flagged patients (six months before study start) was 608/13 097 (4.6%) in intervention practices and 380/11 376 (3.3%) in control practices. During the study 2071 (16%) of flagged patients in the intervention practices and 1163 (10%) in control practices were tested for HCV: overall intervention effect as an adjusted rate ratio of 1.59 (95% confidence interval 1.21 to 2.08; P<0.001). HCV antibodies were detected in 129 patients from intervention practices and 51 patients from control practices (adjusted rate ratio 2.24, 1.47 to 3.42) with weak evidence of an increase in yield (6.2% v 4.4%; adjusted risk ratio 1.40, 0.99 to 1.95). Referral and assessment increased in intervention practices compared with control practices (adjusted rate ratio 5.78, 1.6 to 21.6) with a risk difference of 1.3 per 1000 and a "number needed to help" of one extra HCV diagnosis, referral, and assessment per 792 (95% confidence interval 558 to 1883) patients flagged. The average cost of HCV case finding was £4.03 (95% confidence interval £2.27 to £5.80) per at risk patient and £3165 per additional patient assessed at hepatology. The incremental cost effectiveness ratio was £6212 per quality adjusted life year (QALY), with 92.5% probability of being below £20 000 per QALY.
Conclusion: HepCATT had a modest impact but is a low cost intervention that merits optimisation and implementation as part of an NHS strategy to increase HCV testing and treatment.
Trial registration: ISRCTN61788850.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support for the submitted work as described above;MH has received unrestricted honorariums for presenting at meetings from Abbvie, Gilead, and MSD; PV has received unrestricted honorariums for presenting at meetings from Abbvie and Gilead; no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- Degenhardt L, Charlson F, Stanaway J, et al. . Estimating the burden of disease attributable to injecting drug use as a risk factor for HIV, hepatitis C, and hepatitis B: findings from the Global Burden of Disease Study 2013. Lancet Infect Dis 2016;16:1385-98. 10.1016/S1473-3099(16)30325-5 - DOI - PubMed
-
- Williams R, Aspinall R, Bellis M, et al. . Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet 2014;384:1953-97. 10.1016/S0140-6736(14)61838-9 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
