Trabeculotomy versus combined trabeculotomy-trabeculectomy for primary congenital glaucoma: study protocol of a randomised controlled trial
- PMID: 32102810
- PMCID: PMC7045219
- DOI: 10.1136/bmjopen-2019-032957
Trabeculotomy versus combined trabeculotomy-trabeculectomy for primary congenital glaucoma: study protocol of a randomised controlled trial
Abstract
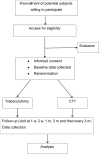
Introduction: Trabeculotomy and combined trabeculotomy-trabeculectomy (CTT) are major surgical options for primary congenital glaucoma (PCG). However, it is unclear which of these two surgical procedures should be recommended as the optimum first-line treatment for PCG. This trial aims to determine whether the outcomes of trabeculotomy are non-inferior to those of CTT in moderate PCG with a horizontal corneal diameter (HCD) of 12-14 mm.
Methods and analysis: This is a 3-year, non-inferiority, prospective, randomised controlled trial. We plan to recruite 248 participants (aged ≤3 years) with PCG with an HCD of 12-14 mm from the Department of Glaucoma, Zhongshan Ophthalmic Center, Guangzhou, China. One eye per participant will be randomly (1:1) assigned to receive trabeculotomy or CTT. The primary outcome is the 3-year postoperative success rate in lowering intraocular pressure (IOP), and the secondary clinical outcomes will include IOP reduction, visual acuity, HCD, central corneal thickness, axial length, cup-disc ratio, refractive error and postoperative complications. Data will be analysed by the intention-to-treat principle.
Ethical approval and dissemination: The study protocol has been approved by the ethics committee of Zhongshan Ophthalmic Center (2014MEKY023) and the '5010 Plan' evaluation committee at Sun Yat-Sen University, Guangzhou, China. The results will be disseminated in international academic meetings and published in peer-reviewed journals.
Trial registration number: Chinese Clinical Trial Registry, ChiCTR-IOR-14005588; Date registered: 20 November 2014.
Keywords: PCG; combined trabeculotomy-trabeculectomy; primary congenital glaucoma; randomised controlled trial; trabeculotomy.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical