Identification and Cloning of a New Western Epstein-Barr Virus Strain That Efficiently Replicates in Primary B Cells
- PMID: 32102884
- PMCID: PMC7199418
- DOI: 10.1128/JVI.01918-19
Identification and Cloning of a New Western Epstein-Barr Virus Strain That Efficiently Replicates in Primary B Cells
Abstract
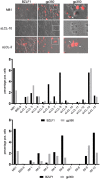
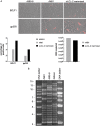
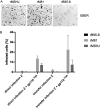
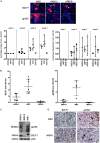
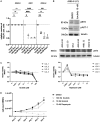
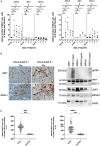
The Epstein-Barr virus (EBV) causes human cancers, and epidemiological studies have shown that lytic replication is a risk factor for some of these tumors. This fits with the observation that EBV M81, which was isolated from a Chinese patient with nasopharyngeal carcinoma, induces potent virus production and increases the risk of genetic instability in infected B cells. To find out whether this property extends to viruses found in other parts of the world, we investigated 22 viruses isolated from Western patients. While one-third of the viruses hardly replicated, the remaining viruses showed variable levels of replication, with three isolates replicating at levels close to that of M81 in B cells. We cloned one strongly replicating virus into a bacterial artificial chromosome (BAC); the resulting recombinant virus (MSHJ) retained the properties of its nonrecombinant counterpart and showed similarities to M81, undergoing lytic replication in vitro and in vivo after 3 weeks of latency. In contrast, B cells infected with the nonreplicating Western B95-8 virus showed early but abortive replication accompanied by cytoplasmic BZLF1 expression. Sequencing confirmed that rMSHJ is a Western virus, being genetically much closer to B95-8 than to M81. Spontaneous replication in rM81- and rMSHJ-infected B cells was dependent on phosphorylated Btk and was inhibited by exposure to ibrutinib, opening the way to clinical intervention in patients with abnormal EBV replication. As rMSHJ contains the complete EBV genome and induces lytic replication in infected B cells, it is ideal to perform genetic analyses of all viral functions in Western strains and their associated diseases.IMPORTANCE The Epstein-Barr virus (EBV) infects the majority of the world population but causes different diseases in different countries. Evidence that lytic replication, the process that leads to new virus progeny, is linked to cancer development is accumulating. Indeed, viruses such as M81 that were isolated from Far Eastern nasopharyngeal carcinomas replicate strongly in B cells. We show here that some viruses isolated from Western patients, including the MSHJ strain, share this property. Moreover, replication of both M81 and of MSHJ was sensitive to ibrutinib, a commonly used drug, thereby opening an opportunity for therapeutic intervention. Sequencing of MSHJ showed that this virus is quite distant from M81 and is much closer to nonreplicating Western viruses. We conclude that Western EBV strains are heterogeneous, with some viruses being able to replicate more strongly and therefore being potentially more pathogenic than others, and that the virus sequence information alone cannot predict this property.
Keywords: Epstein-Barr virus; lytic replication; recombinant virus.
Copyright © 2020 American Society for Microbiology.
Figures




References
-
- Rickinson AB, Kieff E. 2007. Epstein-Barr virus, p 2655–2700. In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Strauss SE (ed). Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Tsai M-H, Raykova A, Klinke O, Bernhardt K, Gärtner K, Leung CS, Geletneky K, Sertel S, Münz C, Feederle R, Delecluse H-J. 2013. Spontaneous lytic replication and epitheliotropism define an Epstein-Barr virus strain found in carcinomas. Cell Rep 5:458–470. doi:10.1016/j.celrep.2013.09.012. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

