Interneuron Desynchronization Precedes Seizures in a Mouse Model of Dravet Syndrome
- PMID: 32102923
- PMCID: PMC7096149
- DOI: 10.1523/JNEUROSCI.2370-19.2020
Interneuron Desynchronization Precedes Seizures in a Mouse Model of Dravet Syndrome
Abstract
Recurrent seizures, which define epilepsy, are transient abnormalities in the electrical activity of the brain. The mechanistic basis of seizure initiation, and the contribution of defined neuronal subtypes to seizure pathophysiology, remains poorly understood. We performed in vivo two-photon calcium imaging in neocortex during temperature-induced seizures in male and female Dravet syndrome (Scn1a+/-) mice, a neurodevelopmental disorder with prominent temperature-sensitive epilepsy. Mean activity of both putative principal cells and parvalbumin-positive interneurons (PV-INs) was higher in Scn1a+/- relative to wild-type controls during quiet wakefulness at baseline and at elevated core body temperature. However, wild-type PV-INs showed a progressive synchronization in response to temperature elevation that was absent in PV-INs from Scn1a+/- mice. Hence, PV-IN activity remains intact interictally in Scn1a+/- mice, yet exhibits decreased synchrony immediately before seizure onset. We suggest that impaired PV-IN synchronization may contribute to the transition to the ictal state during temperature-induced seizures in Dravet syndrome.SIGNIFICANCE STATEMENT Epilepsy is a common neurological disorder defined by recurrent, unprovoked seizures. However, basic mechanisms of seizure initiation and propagation remain poorly understood. We performed in vivo two-photon calcium imaging in an experimental model of Dravet syndrome (Scn1a+/- mice)-a severe neurodevelopmental disorder defined by temperature-sensitive, treatment-resistant epilepsy-and record activity of putative excitatory neurons and parvalbumin-positive GABAergic neocortical interneurons (PV-INs) during naturalistic seizures induced by increased core body temperature. PV-IN activity was higher in Scn1a+/- relative to wild-type controls during quiet wakefulness. However, wild-type PV-INs showed progressive synchronization in response to temperature elevation that was absent in PV-INs from Scn1a+/- mice before seizure onset. Hence, impaired PV-IN synchronization may contribute to transition to seizure in Dravet syndrome.
Keywords: Dravet syndrome; GABAergic interneurons; Nav1.1; epilepsy; seizures; two-photon calcium imaging.
Copyright © 2020 the authors.
Figures


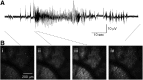


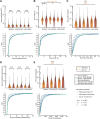
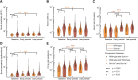

Comment in
-
The Heated Relationship Between Neural Activity and Seizures.Epilepsy Curr. 2020 Dec 8;21(1):62-63. doi: 10.1177/1535759720976371. eCollection 2021 Jan-Feb. Epilepsy Curr. 2020. PMID: 34025277 Free PMC article. No abstract available.
References
-
- Berdyyeva TK, Frady EP, Nassi JJ, Aluisio L, Cherkas Y, Otte S, Wyatt RM, Dugovic C, Ghosh KK, Schnitzer MJ, Lovenberg T, Bonaventure P (2016) Direct imaging of hippocampal epileptiform calcium motifs following kainic acid administration in freely behaving mice. Front Neurosci 10:53. 10.3389/fnins.2016.00053 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
