B38-CAP is a bacteria-derived ACE2-like enzyme that suppresses hypertension and cardiac dysfunction
- PMID: 32103002
- PMCID: PMC7044196
- DOI: 10.1038/s41467-020-14867-z
B38-CAP is a bacteria-derived ACE2-like enzyme that suppresses hypertension and cardiac dysfunction
Abstract
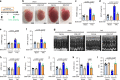
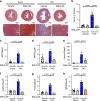
Angiotensin-converting enzyme 2 (ACE2) is critically involved in cardiovascular physiology and pathology, and is currently clinically evaluated to treat acute lung failure. Here we show that the B38-CAP, a carboxypeptidase derived from Paenibacillus sp. B38, is an ACE2-like enzyme to decrease angiotensin II levels in mice. In protein 3D structure analysis, B38-CAP homolog shares structural similarity to mammalian ACE2 with low sequence identity. In vitro, recombinant B38-CAP protein catalyzed the conversion of angiotensin II to angiotensin 1-7, as well as other known ACE2 target peptides. Treatment with B38-CAP suppressed angiotensin II-induced hypertension, cardiac hypertrophy, and fibrosis in mice. Moreover, B38-CAP inhibited pressure overload-induced pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction in mice. Our data identify the bacterial B38-CAP as an ACE2-like carboxypeptidase, indicating that evolution has shaped a bacterial carboxypeptidase to a human ACE2-like enzyme. Bacterial engineering could be utilized to design improved protein drugs for hypertension and heart failure.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Corvol P, Jeunemaitre X, Charru A, Kotelevtsev Y, Soubrier F. Role of the renin-angiotensin system in blood pressure regulation and in human hypertension: new insights from molecular genetics. Recent Prog. Horm. Res. 1995;50:287–308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

