Combining DNMT and HDAC6 inhibitors increases anti-tumor immune signaling and decreases tumor burden in ovarian cancer
- PMID: 32103105
- PMCID: PMC7044433
- DOI: 10.1038/s41598-020-60409-4
Combining DNMT and HDAC6 inhibitors increases anti-tumor immune signaling and decreases tumor burden in ovarian cancer
Erratum in
-
Author Correction: Combining DNMT and HDAC6 inhibitors increases anti-tumor immune signaling and decreases tumor burden in ovarian cancer.Sci Rep. 2021 Dec 20;11(1):24423. doi: 10.1038/s41598-021-04066-1. Sci Rep. 2021. PMID: 34931014 Free PMC article. No abstract available.
Abstract
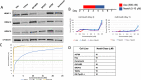
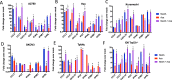
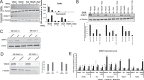
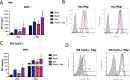
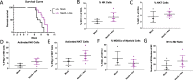
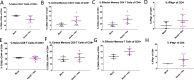
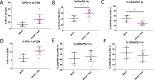
Novel therapies are urgently needed for ovarian cancer, the deadliest gynecologic malignancy. Ovarian cancer has thus far been refractory to immunotherapies that stimulate the host immune system to recognize and kill cancer cells. This may be because of a suppressive tumor immune microenvironment and lack of recruitment and activation of immune cells that kill cancer cells. Our previous work showed that epigenetic drugs including DNA methyltransferase inhibitors and histone deacetylase 6 inhibitors (DNMTis and HDAC6is) individually increase immune signaling in cancer cells. We find that combining DNMTi and HDAC6i results in an amplified type I interferon response, leading to increased cytokine and chemokine expression and higher expression of the MHC I antigen presentation complex in human and mouse ovarian cancer cell lines. Treating mice bearing ID8 Trp53-/- ovarian cancer with HDAC6i/DNMTi led to an increase in tumor-killing cells such as IFNg+ CD8, NK, and NKT cells and a reversal of the immunosuppressive tumor microenvironment with a decrease in MDSCs and PD-1hi CD4 T cells, corresponding with an increase in survival. Thus combining the epigenetic modulators DNMTi and HDAC6i increases anti-tumor immune signaling from cancer cells and has beneficial effects on the ovarian tumor immune microenvironment.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Inhibiting DNA methylation and RNA editing upregulates immunogenic RNA to transform the tumor microenvironment and prolong survival in ovarian cancer.J Immunother Cancer. 2022 Nov;10(11):e004974. doi: 10.1136/jitc-2022-004974. J Immunother Cancer. 2022. PMID: 36343976 Free PMC article.
-
Epigenetic therapy activates type I interferon signaling in murine ovarian cancer to reduce immunosuppression and tumor burden.Proc Natl Acad Sci U S A. 2017 Dec 19;114(51):E10981-E10990. doi: 10.1073/pnas.1712514114. Epub 2017 Dec 4. Proc Natl Acad Sci U S A. 2017. PMID: 29203668 Free PMC article.
-
Combined Trabectedin and anti-PD1 antibody produces a synergistic antitumor effect in a murine model of ovarian cancer.J Transl Med. 2015 Jul 29;13:247. doi: 10.1186/s12967-015-0613-y. J Transl Med. 2015. PMID: 26219551 Free PMC article.
-
Combining epigenetic and immune therapy to overcome cancer resistance.Semin Cancer Biol. 2020 Oct;65:99-113. doi: 10.1016/j.semcancer.2019.12.019. Epub 2019 Dec 23. Semin Cancer Biol. 2020. PMID: 31877341 Free PMC article. Review.
-
A Systematic Review of the Tumor-Infiltrating CD8+ T-Cells/PD-L1 Axis in High-Grade Glial Tumors: Toward Personalized Immuno-Oncology.Front Immunol. 2021 Sep 17;12:734956. doi: 10.3389/fimmu.2021.734956. eCollection 2021. Front Immunol. 2021. PMID: 34603316 Free PMC article.
Cited by
-
Expression of acetylated histones H3 and H4 and histone deacetylase enzymes HDAC1, HDAC2 and HDAC6 in simple mammary carcinomas of female dogs.Front Genet. 2023 Nov 9;14:1257932. doi: 10.3389/fgene.2023.1257932. eCollection 2023. Front Genet. 2023. PMID: 38028583 Free PMC article.
-
Targeting MHC-I molecules for cancer: function, mechanism, and therapeutic prospects.Mol Cancer. 2023 Dec 2;22(1):194. doi: 10.1186/s12943-023-01899-4. Mol Cancer. 2023. PMID: 38041084 Free PMC article. Review.
-
Comprehensive Analysis of DNA Methyltransferases Expression in Primary and Relapsed Ovarian Carcinoma.Cancers (Basel). 2023 Oct 11;15(20):4950. doi: 10.3390/cancers15204950. Cancers (Basel). 2023. PMID: 37894317 Free PMC article.
-
Editorial: Genetic and Epigenetic Control of Immune Responses.Front Immunol. 2021 Oct 5;12:775101. doi: 10.3389/fimmu.2021.775101. eCollection 2021. Front Immunol. 2021. PMID: 34675944 Free PMC article. No abstract available.
-
Histone Deacetylase as a Valuable Predictive Biomarker and Therapeutic Target in Immunotherapy for Non-Small Cell Lung Cancer.Cancer Res Treat. 2022 Apr;54(2):458-468. doi: 10.4143/crt.2021.425. Epub 2021 Sep 10. Cancer Res Treat. 2022. PMID: 34517693 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

