Oral dextrose reduced procedural pain without altering cellular ATP metabolism in preterm neonates: a prospective randomized trial
- PMID: 32103160
- PMCID: PMC7253349
- DOI: 10.1038/s41372-020-0634-0
Oral dextrose reduced procedural pain without altering cellular ATP metabolism in preterm neonates: a prospective randomized trial
Abstract
Objective: To examine the effects of 30% oral dextrose on biochemical markers of pain, adenosine triphosphate (ATP) degradation, and oxidative stress in preterm neonates experiencing a clinically required heel lance.
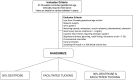
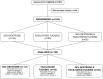
Study design: Utilizing a prospective study design, preterm neonates that met study criteria (n = 169) were randomized to receive either (1) 30% oral dextrose, (2) facilitated tucking, or (3) 30% oral dextrose and facilitated tucking 2 min before heel lance. Plasma markers of ATP degradation (hypoxanthine, uric acid) and oxidative stress (allantoin) were measured before and after the heel lance. Pain was measured using the premature infant pain profile-revised (PIPP-R).
Results: Oral dextrose, administered alone or with facilitated tucking, did not alter plasma markers of ATP utilization and oxidative stress.
Conclusion: A single dose of 30% oral dextrose, given before a clinically required heel lance, decreased signs of pain without increasing ATP utilization and oxidative stress in premature neonates.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous