MUC1 oncoprotein mitigates ER stress via CDA-mediated reprogramming of pyrimidine metabolism
- PMID: 32103170
- PMCID: PMC7165067
- DOI: 10.1038/s41388-020-1225-4
MUC1 oncoprotein mitigates ER stress via CDA-mediated reprogramming of pyrimidine metabolism
Abstract
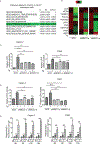
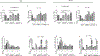
The Mucin 1 (MUC1) protein is overexpressed in various cancers and mediates chemotherapy resistance. However, the mechanism is not fully understood. Given that most chemotherapeutic drugs disrupt ER homeostasis as part of their toxicity, and MUC1 expression is regulated by proteins involved in ER homeostasis, we investigated the link between MUC1 and ER homeostasis. MUC1 knockdown in pancreatic cancer cells enhanced unfolded protein response (UPR) signaling and cell death upon ER stress induction. Transcriptomic analysis revealed alterations in the pyrimidine metabolic pathway and cytidine deaminase (CDA). ChIP and CDA activity assays showed that MUC1 occupied CDA gene promoter upon ER stress induction correlating with increased CDA expression and activity in MUC1-expressing cells as compared with MUC1 knockdown cells. Inhibition of either the CDA or pyrimidine metabolic pathway diminished survival in MUC1-expressing cancer cells upon ER stress induction. Metabolomic analysis demonstrated that MUC1-mediated CDA activity corresponded to deoxycytidine to deoxyuridine metabolic reprogramming upon ER stress induction. The resulting increase in deoxyuridine mitigated ER stress-induced cytotoxicity. In addition, given (1) the established roles of MUC1 in protecting cells against reactive oxygen species (ROS) insults, (2) ER stress-generated ROS further promote ER stress and (3) the emerging anti-oxidant property of deoxyuridine, we further investigated if MUC1 regulated ER stress by a deoxyuridine-mediated modulation of ROS levels. We observed that deoxyuridine could abrogate ROS-induced ER stress to promote cancer cell survival. Taken together, our findings demonstrate a novel MUC1-CDA axis of the adaptive UPR that provides survival advantage upon ER stress induction.
Conflict of interest statement
Figures

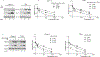


References
-
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nature reviews Molecular cell biology. 2003;4(7):517–29. - PubMed
-
- Ikonen E Cellular cholesterol trafficking and compartmentalization. Nature reviews Molecular cell biology. 2008;9(2):125–38. - PubMed
-
- Jun HS, Lee YM, Cheung YY, McDermott DH, Murphy PM, De Ravin SS, et al. Lack of glucose recycling between endoplasmic reticulum and cytoplasm underlies cellular dysfunction in glucose-6-phosphatase-beta-deficient neutrophils in a congenital neutropenia syndrome. Blood. 2010;116(15):2783–92. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

