Sex-specific adipose tissue imprinting of regulatory T cells
- PMID: 32103173
- PMCID: PMC7241647
- DOI: 10.1038/s41586-020-2040-3
Sex-specific adipose tissue imprinting of regulatory T cells
Erratum in
-
Author Correction: Sex-specific adipose tissue imprinting of regulatory T cells.Nature. 2021 Mar;591(7851):E26. doi: 10.1038/s41586-020-2251-7. Nature. 2021. PMID: 33674801 No abstract available.
Abstract
Adipose tissue is an energy store and a dynamic endocrine organ1,2. In particular, visceral adipose tissue (VAT) is critical for the regulation of systemic metabolism3,4. Impaired VAT function-for example, in obesity-is associated with insulin resistance and type 2 diabetes5,6. Regulatory T (Treg) cells that express the transcription factor FOXP3 are critical for limiting immune responses and suppressing tissue inflammation, including in the VAT7-9. Here we uncover pronounced sexual dimorphism in Treg cells in the VAT. Male VAT was enriched for Treg cells compared with female VAT, and Treg cells from male VAT were markedly different from their female counterparts in phenotype, transcriptional landscape and chromatin accessibility. Heightened inflammation in the male VAT facilitated the recruitment of Treg cells via the CCL2-CCR2 axis. Androgen regulated the differentiation of a unique IL-33-producing stromal cell population specific to the male VAT, which paralleled the local expansion of Treg cells. Sex hormones also regulated VAT inflammation, which shaped the transcriptional landscape of VAT-resident Treg cells in a BLIMP1 transcription factor-dependent manner. Overall, we find that sex-specific differences in Treg cells from VAT are determined by the tissue niche in a sex-hormone-dependent manner to limit adipose tissue inflammation.
Conflict of interest statement
Figures








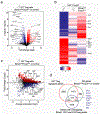




References
-
- Meseguer A, Puche C & Cabero A Sex steroid biosynthesis in white adipose tissue. Horm Metab Res 34, 731–736 (2002). - PubMed
-
- Kamat A, Hinshelwood MM, Murry BA & Mendelson CR Mechanisms in tissue-specific regulation of estrogen biosynthesis in humans. Trends Endocrinol Metab 13, 122–128 (2002). - PubMed
-
- Mraz M & Haluzik M The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrinol 222, R113–127 (2014). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

