Understanding the role of antibody glycosylation through the lens of severe viral and bacterial diseases
- PMID: 32103252
- PMCID: PMC7109349
- DOI: 10.1093/glycob/cwaa018
Understanding the role of antibody glycosylation through the lens of severe viral and bacterial diseases
Abstract
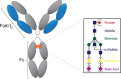
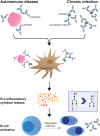
Abundant evidence points to a critical role for antibodies in protection and pathology across infectious diseases. While the antibody variable domain facilitates antibody binding and the blockade of infection, the constant domain (Fc) mediates cross talk with the innate immune system. The biological activity of the Fc region is controlled genetically via class switch recombination, resulting in the selection of distinct antibody isotypes and subclasses. However, a second modification is made to all antibodies, via post-translational changes in antibody glycosylation. Studies from autoimmunity and oncology have established the role of immunoglobulin G (IgG) Fc glycosylation as a key regulator of humoral immune activity. However, a growing body of literature, exploring IgG Fc glycosylation through the lens of infectious diseases, points to the role of inflammation in shaping Fc-glycan profiles, the remarkable immune plasticity in antibody glycosylation across pathogen-exposed populations, the canonical and noncanonical functions of glycans and the existence of antigen-specific control over antibody Fc glycosylation. Ultimately, this work provides critical new insights into the functional roles for antibody glycosylation as well as lays the foundation for leveraging antibody glycosylation to drive prevention or control across diseases.
Keywords: Fc; antibody; glycosylation; humoral immunity; infectious disease.
© The Author(s) 2020. Published by Oxford University Press.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

