Comparison of the efficacy of MOE and PMO modifications of systemic antisense oligonucleotides in a severe SMA mouse model
- PMID: 32103257
- PMCID: PMC7102994
- DOI: 10.1093/nar/gkaa126
Comparison of the efficacy of MOE and PMO modifications of systemic antisense oligonucleotides in a severe SMA mouse model
Abstract
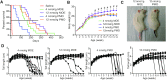
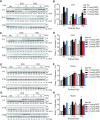
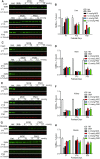
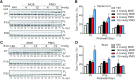
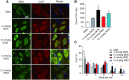
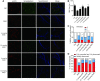
Spinal muscular atrophy (SMA) is a motor neuron disease. Nusinersen, a splice-switching antisense oligonucleotide (ASO), was the first approved drug to treat SMA. Based on prior preclinical studies, both 2'-O-methoxyethyl (MOE) with a phosphorothioate backbone and morpholino with a phosphorodiamidate backbone-with the same or extended target sequence as nusinersen-displayed efficient rescue of SMA mouse models. Here, we compared the therapeutic efficacy of these two modification chemistries in rescue of a severe mouse model using ASO10-29-a 2-nt longer version of nusinersen-via subcutaneous injection. Although both chemistries efficiently corrected SMN2 splicing in various tissues, restored motor function and improved the integrity of neuromuscular junctions, MOE-modified ASO10-29 (MOE10-29) was more efficacious than morpholino-modified ASO10-29 (PMO10-29) at the same molar dose, as seen by longer survival, greater body-weight gain and better preservation of motor neurons. Time-course analysis revealed that MOE10-29 had more persistent effects than PMO10-29. On the other hand, PMO10-29 appears to more readily cross an immature blood-brain barrier following systemic administration, showing more robust initial effects on SMN2 exon 7 inclusion, but less persistence in the central nervous system. We conclude that both modifications can be effective as splice-switching ASOs in the context of SMA and potentially other diseases, and discuss the advantages and disadvantages of each.
© The Author(s) 2020. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


Similar articles
-
Effect of combined systemic and local morpholino treatment on the spinal muscular atrophy Δ7 mouse model phenotype.Clin Ther. 2014 Mar 1;36(3):340-56.e5. doi: 10.1016/j.clinthera.2014.02.004. Clin Ther. 2014. PMID: 24636820
-
Antisense correction of SMN2 splicing in the CNS rescues necrosis in a type III SMA mouse model.Genes Dev. 2010 Aug 1;24(15):1634-44. doi: 10.1101/gad.1941310. Epub 2010 Jul 12. Genes Dev. 2010. PMID: 20624852 Free PMC article.
-
Morpholino-Mediated Exon Inclusion for SMA.Methods Mol Biol. 2018;1828:467-477. doi: 10.1007/978-1-4939-8651-4_29. Methods Mol Biol. 2018. PMID: 30171560
-
Challenges and future perspective of antisense therapy for spinal muscular atrophy: A review.Eur J Cell Biol. 2023 Jun;102(2):151326. doi: 10.1016/j.ejcb.2023.151326. Epub 2023 Jun 2. Eur J Cell Biol. 2023. PMID: 37295266 Review.
-
Nusinersen: antisense oligonucleotide to increase SMN protein production in spinal muscular atrophy.Drugs Today (Barc). 2017 Jun;53(6):327-337. doi: 10.1358/dot.2017.53.6.2652413. Drugs Today (Barc). 2017. PMID: 28799578 Review.
Cited by
-
RNAi-mediated silencing of SOD1 profoundly extends survival and functional outcomes in ALS mice.bioRxiv [Preprint]. 2024 Jun 25:2024.06.20.599943. doi: 10.1101/2024.06.20.599943. bioRxiv. 2024. Update in: Mol Ther. 2025 Aug 6;33(8):3917-3938. doi: 10.1016/j.ymthe.2025.05.010. PMID: 38979291 Free PMC article. Updated. Preprint.
-
Transport Oligonucleotides-A Novel System for Intracellular Delivery of Antisense Therapeutics.Molecules. 2020 Aug 11;25(16):3663. doi: 10.3390/molecules25163663. Molecules. 2020. PMID: 32796768 Free PMC article.
-
ASO targeting RBM3 temperature-controlled poison exon splicing prevents neurodegeneration in vivo.EMBO Mol Med. 2023 May 8;15(5):e17157. doi: 10.15252/emmm.202217157. Epub 2023 Mar 22. EMBO Mol Med. 2023. PMID: 36946385 Free PMC article.
-
Enhancing Antisense Oligonucleotide-Based Therapeutic Delivery with DG9, a Versatile Cell-Penetrating Peptide.Cells. 2023 Oct 2;12(19):2395. doi: 10.3390/cells12192395. Cells. 2023. PMID: 37830609 Free PMC article. Review.
-
Evolution of antisense oligonucleotides: navigating nucleic acid chemistry and delivery challenges.Expert Opin Drug Discov. 2025 Jan;20(1):63-80. doi: 10.1080/17460441.2024.2440095. Epub 2024 Dec 17. Expert Opin Drug Discov. 2025. PMID: 39653607 Review.
References
-
- De Clercq E., Eckstein E., Merigan T.C.. [Interferon induction increased through chemical modification of a synthetic polyribonucleotide]. Science (New York, N.Y.). 1969; 165:1137–1139. - PubMed
-
- Teplova M., Minasov G., Tereshko V., Inamati G.B., Cook P.D., Manoharan M., Egli M.. Crystal structure and improved antisense properties of 2′-O-(2-methoxyethyl)-RNA. Nat. Struct. Biol. 1999; 6:535–539. - PubMed
-
- Seth P.P., Siwkowski A., Allerson C.R., Vasquez G., Lee S., Prakash T.P., Kinberger G., Migawa M.T., Gaus H., Bhat B. et al. .. Design, synthesis and evaluation of constrained methoxyethyl (cMOE) and constrained ethyl (cEt) nucleoside analogs. Nucleic Acids Symp. Ser. 2008; 2004:553–554. - PubMed
-
- Kumar R., Singh S.K., Koshkin A.A., Rajwanshi V.K., Meldgaard M., Wengel J.. The first analogues of LNA (locked nucleic acids): phosphorothioate-LNA and 2′-thio-LNA. Bioorg. Med. Chem. Lett. 1998; 8:2219–2222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

