Incidence and frequency of cancer cachexia during chemotherapy for advanced pancreatic ductal adenocarcinoma
- PMID: 32103356
- PMCID: PMC7546994
- DOI: 10.1007/s00520-020-05346-8
Incidence and frequency of cancer cachexia during chemotherapy for advanced pancreatic ductal adenocarcinoma
Abstract
Purpose: Cachexia influences the patient's physical wellbeing and quality of life, and the patient's ability to tolerate their cancer therapies, especially cytotoxic chemotherapy. The purpose of this study was to investigate the frequency and timing of onset of cancer cachexia during chemotherapy and its association with prognosis and toxicity in patients with pancreatic ductal adenocarcinoma (PDAC).
Methods: We performed a retrospective study in patients who underwent first-line chemotherapy after diagnosis of advanced PDAC between 6 June 2008 and 31 March 2017. Base cachexia (weight loss up to 6 months before starting first-line chemotherapy) and follow-up cachexia (after starting first-line chemotherapy) were defined as weight loss > 2% with a body mass index (BMI) < 20 kg/m2 or weight loss > 5%.
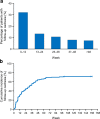
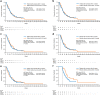
Results: A total of 150 patients were registered. The median age and BMI were 65 years and 21.7 kg/m2, respectively. Base cachexia occurred in 50% of patients. Follow-up cachexia occurred in 32% within 12 weeks of starting first-line chemotherapy, reaching 64% at 1 year. Overall survival was not significantly different between patients with and without follow-up cachexia, regardless of whether cancer cachexia occurred within 12, 24, or 48 weeks of starting first-line treatment. Appetite loss, fatigue, nausea, and diarrhea were more frequent in patients with follow-up cachexia than in those without follow-up cachexia.
Conclusion: Follow-up cachexia had an early onset, but was not a prognostic factor for overall survival in patients with PDAC. Some adverse events tended to be more frequent in patients with follow-up cachexia than in those without follow-up cachexia.
Keywords: Adverse events; Cachexia; Incidence; Pancreatic cancer; Survival.
Conflict of interest statement
Shuichi Mitsunaga has received research funds from Ono Pharmaceutical Co., Ltd. Eiji Kasamatsu and Koji Machii are employees of Ono Pharmaceutical Co., Ltd.
Figures


References
-
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/s1470-2045(10)70218-7. - DOI - PubMed
-
- Escamilla DM, Jarrett P. The impact of weight loss on patients with cancer. Nurs Times. 2016;112:20–22. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

