Effects of a ketogenic diet in overweight women with polycystic ovary syndrome
- PMID: 32103756
- PMCID: PMC7045520
- DOI: 10.1186/s12967-020-02277-0
Effects of a ketogenic diet in overweight women with polycystic ovary syndrome
Abstract
Background: Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women during reproductive age. It is characterised clinically by oligo-ovulation or anovulation, hyper-androgenism, and the presence of polycystic ovaries. It is associated with an increased prevalence of metabolic syndrome, cardiovascular disease and type 2 diabetes. The onset of PCOS has been associated to several hereditary and environmental factors, but insulin resistance plays a key pathogenetic role. We sought to investigate the effects of a ketogenic diet (KD) on women of childbearing age with a diagnosis of PCOS.
Methods: Fourteen overweight women with diagnosis of PCOS underwent to a ketogenic Mediterranean diet with phyoextracts (KEMEPHY) for 12 week. Changes in body weight, body mass index (BMI), fat body mass (FBM), lean body mass (LBM), visceral adipose tissue (VAT), insulin, glucose, HOMA-IR, total cholesterol, low density lipoprotein (LDL), high density lipoprotein (HDL), triglycerides (TGs), total and free testosterone, luteinizing hormone (LH), follicle stimulating hormone (FSH); dehydroepiandrosterone sulfate (DHEAs), estradiol, progesterone, sex hormone binding globulin (SHBG) and Ferriman Gallwey score were evaluated.
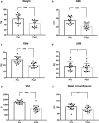
Results: After 12 weeks, anthropometric and body composition measurements revealed a significant reduction of body weight (- 9.43 kg), BMI (- 3.35), FBM (8.29 kg) and VAT. There was a significant, slightly decrease of LBM. A significant decrease in glucose and insulin blood levels were observed, together with a significant improvement of HOMA-IR. A significant decrease of triglycerides, total cholesterol and LDL were observed along with a rise in HDL levels. The LH/FSH ratio, LH total and free testosterone, and DHEAS blood levels were also significantly reduced. Estradiol, progesterone and SHBG increased. The Ferriman Gallwey Score was slightly, although not significantly, reduced.
Conclusions: Our results suggest that a KD may be considered as a valuable non pharmacological treatment for PCOS. Longer treatment periods should be tested to verify the effect of a KD on the dermatological aspects of PCOS. Trial registration Clinicaltrial.gov, NCT04163120, registrered 10 November 2019, retrospectively registered, https://clinicaltrials.gov.
Keywords: Hyperinsulinemia; Ketogenic diet; Ketone bodies; LCKD; Low carbohydrate diet; Overweight; PCOS.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

