Signal recovery from stimulation artifacts in intracranial recordings with dictionary learning
- PMID: 32103828
- PMCID: PMC7333778
- DOI: 10.1088/1741-2552/ab7a4f
Signal recovery from stimulation artifacts in intracranial recordings with dictionary learning
Abstract
Objective: Electrical stimulation of the human brain is commonly used for eliciting and inhibiting neural activity for clinical diagnostics, modifying abnormal neural circuit function for therapeutics, and interrogating cortical connectivity. However, recording electrical signals with concurrent stimulation results in dominant electrical artifacts that mask the neural signals of interest. Here we develop a method to reproducibly and robustly recover neural activity during concurrent stimulation. We concentrate on signal recovery across an array of electrodes without channel-wise fine-tuning of the algorithm. Our goal includes signal recovery with trains of stimulation pulses, since repeated, high-frequency pulses are often required to induce desired effects in both therapeutic and research domains. We have made all of our code and data publicly available.
Approach: We developed an algorithm that automatically detects templates of artifacts across many channels of recording, creating a dictionary of learned templates using unsupervised clustering. The artifact template that best matches each individual artifact pulse is subtracted to recover the underlying activity. To assess the success of our method, we focus on whether it extracts physiologically interpretable signals from real recordings.
Main results: We demonstrate our signal recovery approach on invasive electrophysiologic recordings from human subjects during stimulation. We show the recovery of meaningful neural signatures in both electrocorticographic (ECoG) arrays and deep brain stimulation (DBS) recordings. In addition, we compared cortical responses induced by the stimulation of primary somatosensory (S1) by natural peripheral touch, as well as motor cortex activity with and without concurrent S1 stimulation.
Significance: Our work will enable future advances in neural engineering with simultaneous stimulation and recording.
Figures







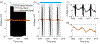




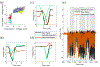
References
-
- Aghabozorgi S, Seyed Shirkhorshidi A and Ying Wah T 2015. Time-series clustering—A decade review Inf. Sys 53 16–38
-
- Al-ani T, Cazettes F, Palfi S and Lefaucheur JP 2011. Automatic removal of high-amplitude stimulus artefact from neuronal signal recorded in the subthalamic nucleus J. Neurosci. Methods 198 135–46 - PubMed
-
- Alagapan S, Shin HW, Fröhlich F and Wu H-t 2018. Diffusion geometry approach to efficiently remove electrical stimulation artifacts in intracranial electroencephalography J. Neural Eng 16 036010. - PubMed
-
- Ashkan K, Rogers P, Bergman H and Ughratdar I 2017. Insights into the mechanisms of deep brain stimulation Nat. Rev. Neurol 13 548–54 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
