The Antiallodynic Effect of Nefopam on Vincristine-Induced Neuropathy in Mice
- PMID: 32104054
- PMCID: PMC7012248
- DOI: 10.2147/JPR.S224478
The Antiallodynic Effect of Nefopam on Vincristine-Induced Neuropathy in Mice
Abstract
Background: Chemotherapy-induced neuropathic pain is a disabling condition following cancer treatment. Vincristine has more neurotoxicity than other vinca alkaloid agents. This study evaluated the correlation of different doses of nefopam with antiallodynic effects in a mouse vincristine neuropathy model.
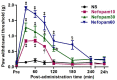
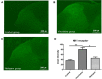
Methods: A peripheral neuropathic mouse model was made by intraperitoneal injection of vincristine (0.1 mg/kg/day; 5-day-on, 2-day-off schedule over 12 days). After the development of allodynia, mice were injected intraperitoneally with 0.9% normal saline (NS group) or various doses (10, 30, 60 mg/kg) of nefopam (Nefopam group). We examined allodynia using von Frey hairs pre-administration and at 30, 60, 90, 120, 180, 240 mins, and 24 hrs after drug administration. We also measured the neurokinin-1 receptor concentrations in the spinal cord to confirm the antiallodynic effect of nefopam after drug administration.
Results: The peripheral neuropathic mouse model showed prominent mechanical allodynia. Intraperitoneal nefopam produced a clear dose-dependent increase in paw withdrawal threshold compared with pre-administration values and versus the NS group. The concentration of neurokinin-1 receptor was significantly decreased in the Nefopam group (P<0.05).
Conclusion: Intraperitoneally administered nefopam yielded a dose-dependent attenuation of mechanical allodynia and decreased neurokinin-1 receptor concentration, suggesting that the neurokinin-1 receptor is involved in the antiallodynic effects of nefopam in vincristine neuropathy.
Keywords: allodynia; mice; nefopam; neurokinin-1; neuropathy; vincristine.
© 2020 Lee et al.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Hou S, Huh B, Kim HK, Kim KH, Abdi S. Treatment of chemotherapy-induced peripheral neuropathy: systematic review and recommendations. Pain Physician. 2018;21(6):571–592. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

