miR-584 inhibits cell proliferation, migration and invasion in vitro and enhances the sensitivity to cisplatin in human cervical cancer by negatively targeting GLI1
- PMID: 32104266
- PMCID: PMC7027228
- DOI: 10.3892/etm.2020.8449
miR-584 inhibits cell proliferation, migration and invasion in vitro and enhances the sensitivity to cisplatin in human cervical cancer by negatively targeting GLI1
Abstract
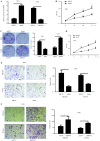
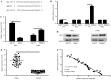
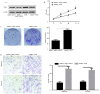
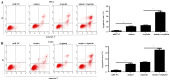
Cervical cancer is the most lethal malignancy amongst women worldwide. MicroRNAs (miRNAs/miRs) play a critical role in the progression of cervical cancer. Compelling evidence indicates that miR-584 acts as a tumor suppressor in some types of cancers. However, the function of miR-584 in cervical cancer has not been illustrated. In the present study, the effects and mechanism of miR-584 in the process of proliferation, migration and invasion, and drug sensitivity to cisplatin in cervical cancer were determined. miR-584 expression decreased markedly in cervical cancer tissues and cell lines compared with healthy control samples. Dual-luciferase reporter assays confirmed that glioma-associated oncogene 1 (GLI1) is a novel molecular target of miR-584. The overexpression of miR-584 inhibited the expression of GLI1, reduced cell proliferation, migration and invasion, and induced apoptosis in HeLa cells. However, the silencing of miR-584 in CaSki cells produced the opposite effects. In addition, the overexpression of GLI1 in HeLa-cells overexpressing miR-584 markedly reversed the miR-584-induced inhibitory effect. Flow cytometry results showed that miR-584 enhanced cisplatin sensitivity by promoting chemotherapy-induced apoptosis. Therefore, miR-584 acted as a tumor suppressor miRNA and might be a novel target gene for future cervical cancer treatments.
Keywords: cervical cancer; cisplatin; glioma-associated oncogene 1; microRNA-584; migration and invasion; proliferation.
Copyright: © Wang et al.
Figures

References
LinkOut - more resources
Full Text Sources
