Atorvastatin promotes AMPK signaling to protect against high fat diet-induced non-alcoholic fatty liver in golden hamsters
- PMID: 32104276
- PMCID: PMC7027324
- DOI: 10.3892/etm.2020.8465
Atorvastatin promotes AMPK signaling to protect against high fat diet-induced non-alcoholic fatty liver in golden hamsters
Abstract
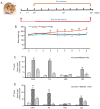
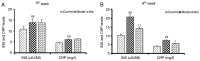
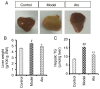
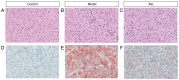
Non-alcoholic fatty liver disease (NAFLD) is characterized by diffuse fatty acid degeneration and excess fat accumulation in the liver. Notably, the currently available medications used to treat NAFLD remain limited. The aim of the present study was to investigate the protective role of atorvastatin (Ato) against NAFLD in golden hamsters fed a high fat diet (HFD) and in HepG2 cells treated with palmitate, and identify the underlying molecular mechanism. Ato (3 mg/kg) was administered orally every day for 8 weeks to the hamsters during HFD administration. Hamsters in the model group developed hepatic steatosis with high serum levels of triglyceride, cholesterol, insulin and C-reactive protein, which were effectively reduced by treatment with Ato. Additionally, the relative liver weight of hamsters treated with Ato was markedly lower compared with that of the model group. Hematoxylin and eosin, and oil red O staining indicated that the livers of the animals in the model group exhibited large and numerous lipid droplets, which were markedly decreased after Ato treatment. Western blot analysis indicated that Ato inhibited fat accumulation in the liver through the AMP-activated protein kinase (AMPK)-dependent activation of peroxisome proliferator activated receptor α (PPARα), peroxisome proliferator-activated receptor-γ coactivator 1 α and their target genes. Furthermore, in vitro, Ato inhibited PA-induced lipid accumulation in HepG2 cells. This inhibitory effect was attenuated following Compound C treatment, indicating that AMPK may be a potential target of Ato. In conclusion, the increase in AMPK-mediated PPARα and its target genes may represent a novel molecular mechanism by which Ato prevents NAFLD.
Keywords: AMP-activated protein kinase; atorvastatin; lipid accumulation; lipolysis; non-alcoholic fatty liver.
Copyright: © Zhang et al.
Figures


Similar articles
-
Comparison of dietary control and atorvastatin on high fat diet induced hepatic steatosis and hyperlipidemia in rats.Lipids Health Dis. 2011 Jan 26;10:23. doi: 10.1186/1476-511X-10-23. Lipids Health Dis. 2011. PMID: 21269482 Free PMC article.
-
LB100 ameliorates nonalcoholic fatty liver disease via the AMPK/Sirt1 pathway.World J Gastroenterol. 2019 Dec 7;25(45):6607-6618. doi: 10.3748/wjg.v25.i45.6607. World J Gastroenterol. 2019. PMID: 31832001 Free PMC article.
-
Monascin and ankaflavin act as natural AMPK activators with PPARα agonist activity to down-regulate nonalcoholic steatohepatitis in high-fat diet-fed C57BL/6 mice.Food Chem Toxicol. 2014 Feb;64:94-103. doi: 10.1016/j.fct.2013.11.015. Epub 2013 Nov 22. Food Chem Toxicol. 2014. PMID: 24275089
-
Lycopus lucidus Turcz. ex Benth. Attenuates free fatty acid-induced steatosis in HepG2 cells and non-alcoholic fatty liver disease in high-fat diet-induced obese mice.Phytomedicine. 2019 Mar 1;55:14-22. doi: 10.1016/j.phymed.2018.07.008. Epub 2018 Jul 18. Phytomedicine. 2019. PMID: 30668424
-
Vitexin alleviates non-alcoholic fatty liver disease by activating AMPK in high fat diet fed mice.Biochem Biophys Res Commun. 2019 Oct 29;519(1):106-112. doi: 10.1016/j.bbrc.2019.08.139. Epub 2019 Aug 29. Biochem Biophys Res Commun. 2019. PMID: 31472955
Cited by
-
Effect of atorvastatin on lipoxygenase pathway-related gene expression in an in vitro model of lipid accumulation in hepatocytes.FEBS Open Bio. 2023 Apr;13(4):606-616. doi: 10.1002/2211-5463.13552. Epub 2023 Mar 9. FEBS Open Bio. 2023. PMID: 36637998 Free PMC article.
-
Temporal Development of Dyslipidemia and Nonalcoholic Fatty Liver Disease (NAFLD) in Syrian Hamsters Fed a High-Fat, High-Fructose, High-Cholesterol Diet.Nutrients. 2021 Feb 12;13(2):604. doi: 10.3390/nu13020604. Nutrients. 2021. PMID: 33673227 Free PMC article.
-
Atorvastatin on Treatment of Nonalcoholic Fatty Liver Disease Patients.Chonnam Med J. 2024 Jan;60(1):13-20. doi: 10.4068/cmj.2024.60.1.13. Epub 2024 Jan 25. Chonnam Med J. 2024. PMID: 38304133 Free PMC article. Review.
-
The Hypolipidemic Characteristics of a Methanol Extract of Fermented Green Tea and Spore of Eurotium cristatum SXHBTBU1934 in Golden Hamsters.Nutrients. 2023 Mar 8;15(6):1329. doi: 10.3390/nu15061329. Nutrients. 2023. PMID: 36986059 Free PMC article.
-
Safety, Immunogenicity and Protective Activity of a Modified Trivalent Live Attenuated Influenza Vaccine for Combined Protection Against Seasonal Influenza and COVID-19 in Golden Syrian Hamsters.Vaccines (Basel). 2024 Nov 21;12(12):1300. doi: 10.3390/vaccines12121300. Vaccines (Basel). 2024. PMID: 39771962 Free PMC article.
References
-
- Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397.e310. doi: 10.1053/j.gastro.2015.04.043. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
