Gremlin mediates the TGF-β-induced induction of profibrogenic genes in human retinal pigment epithelial cells
- PMID: 32104303
- PMCID: PMC7027231
- DOI: 10.3892/etm.2020.8463
Gremlin mediates the TGF-β-induced induction of profibrogenic genes in human retinal pigment epithelial cells
Abstract
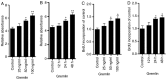
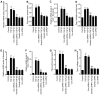
Proliferative vitreoretinopathy (PVR) is characterised by the contraction and growth of fibrotic membranes on the retina and within the vitreous body. Retinal pigment epithelial (RPE) cells, a major cellular component of the fibrotic membrane, is one of the cell types that have been previously reported to associate with PVR pathogenesis. During PVR, RPE cells undergo increased cell proliferation, migration and the secretion of extracellular matrix molecules, such as fibronectin and type I collagen. A variety of cytokines and growth factors are involved in the formation of the fibrotic membrane. Although gremlin has been reported to serve an important role in the regulation of epithelial-to-mesenchymal transition in PVR, the relationship between gremlin and the expression of profibrogenic factors in human RPE cells remains unclear. In the present study, gremlin promoted RPE cell proliferation and the expression of type I collagen and fibronectin. In addition, knocking down gremlin expression by siRNA significantly suppressed the transforming growth factor (TGF)-β1- and TGF-β2-induced expression of type I collagen and fibronectin in RPE cells. These findings suggest that gremlin may serve an important role in the development of PVR.
Keywords: collagen I; fibronectin; gremlin; proliferative vitreoretinopathy; retinal pigment epithelial cells; transforming growth factor-β.
Copyright: © Qin et al.
Figures


Similar articles
-
Protective Effects of Fucoidan on Epithelial-Mesenchymal Transition of Retinal Pigment Epithelial Cells and Progression of Proliferative Vitreoretinopathy.Cell Physiol Biochem. 2018;46(4):1704-1715. doi: 10.1159/000489246. Epub 2018 Apr 23. Cell Physiol Biochem. 2018. PMID: 29698960
-
Gremlin-1: An endogenous BMP antagonist induces epithelial-mesenchymal transition and interferes with redifferentiation in fetal RPE cells with repeated wounds.Mol Vis. 2019 Oct 21;25:625-635. eCollection 2019. Mol Vis. 2019. PMID: 31700227 Free PMC article.
-
BMP7 antagonizes proliferative vitreoretinopathy through retinal pigment epithelial fibrosis in vivo and in vitro.FASEB J. 2019 Mar;33(3):3212-3224. doi: 10.1096/fj.201800858RR. Epub 2018 Nov 1. FASEB J. 2019. PMID: 30383450
-
Blockade of Jagged/Notch pathway abrogates transforming growth factor β2-induced epithelial-mesenchymal transition in human retinal pigment epithelium cells.Curr Mol Med. 2014 May;14(4):523-34. doi: 10.2174/1566524014666140331230411. Curr Mol Med. 2014. PMID: 24694299 Review.
-
[Periostin in the Pathogenesis of Proliferative Vitreoretinopathy].Nippon Ganka Gakkai Zasshi. 2015 Nov;119(11):772-80. Nippon Ganka Gakkai Zasshi. 2015. PMID: 26685481 Review. Japanese.
Cited by
-
Inhibition of epithelial-mesenchymal transition in retinal pigment epithelial cells by a retinoic acid receptor-α agonist.Sci Rep. 2021 Jun 4;11(1):11842. doi: 10.1038/s41598-021-90618-4. Sci Rep. 2021. PMID: 34088917 Free PMC article.
-
Bone morphogenetic protein-6 suppresses TGF-β2-induced epithelial-mesenchymal transition in retinal pigment epithelium.Int J Ophthalmol. 2024 Apr 18;17(4):646-652. doi: 10.18240/ijo.2024.04.06. eCollection 2024. Int J Ophthalmol. 2024. PMID: 38638261 Free PMC article.
-
Fibrotic Changes in Rhegmatogenous Retinal Detachment.Int J Mol Sci. 2025 Jan 25;26(3):1025. doi: 10.3390/ijms26031025. Int J Mol Sci. 2025. PMID: 39940795 Free PMC article. Review.
-
Gremlin: a complex molecule regulating wound healing and fibrosis.Cell Mol Life Sci. 2021 Dec;78(24):7917-7923. doi: 10.1007/s00018-021-03964-x. Epub 2021 Nov 3. Cell Mol Life Sci. 2021. PMID: 34731251 Free PMC article. Review.
References
-
- Pena RA, Jerdan JA, Glaser BM. Effects of TGF-beta and TGF-beta neutralizing antibodies on fibroblast-induced collagen gel contraction: Implications for proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1994;35:2804–2808. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
