Preparation of CaP/pDNA nanoparticles by reverse micro-emulsion method: Optimization of formulation variables using experimental design
- PMID: 32104328
- PMCID: PMC7032106
- DOI: 10.1016/j.ajps.2016.09.006
Preparation of CaP/pDNA nanoparticles by reverse micro-emulsion method: Optimization of formulation variables using experimental design
Abstract
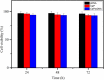
In this study, the CaP/pDNA nanoparticles were prepared using Triton X-100/Butanol/Cyclohexane/Water reverse microemulsion system. Optimization of preparation conditions was based on evaluation of particle size by Box-Behnken design method. The particle sizes of the optimized CaP/pDNA nanoparticles were found to be 60.23 ± 4.72 nm, polydispersity index was 0.252 and pDNA encapsulate efficiency was more than 90%. The optimized CaP/pDNA nanoparticles have pH sensitivity and biocompatibility. Further, optimized CaP/pDNA nanoparticles showed higher transfection efficiency.
Keywords: Box–Behnken design; CaP nanoparticles; Reverse microemulsion method; Transfection and expression; pDNA.
© 2017 Shenyang Pharmaceutical University. Production and hosting by Elsevier B.V.
Figures




Similar articles
-
Preparation of Calcium Phosphate/pDNA Nanoparticles for Exogenous Gene Delivery by Co-Precipitation Method: Optimization of Formulation Variables Using Box-Behnken Design.J Pharm Sci. 2017 Aug;106(8):2053-2059. doi: 10.1016/j.xphs.2017.04.049. Epub 2017 May 5. J Pharm Sci. 2017. PMID: 28483423
-
Plasmid DNA-entrapped nanoparticles engineered from microemulsion precursors: in vitro and in vivo evaluation.Bioconjug Chem. 2002 Nov-Dec;13(6):1319-27. doi: 10.1021/bc0255586. Bioconjug Chem. 2002. PMID: 12440869
-
Calcium phosphate embedded PLGA nanoparticles: a promising gene delivery vector with high gene loading and transfection efficiency.Int J Pharm. 2012 Jul 15;431(1-2):210-21. doi: 10.1016/j.ijpharm.2012.04.046. Epub 2012 Apr 23. Int J Pharm. 2012. PMID: 22561795
-
Evaluation of the high-pressure extrusion technique as a method for sizing plasmid DNA-containing cationic liposomes.J Liposome Res. 2011 Dec;21(4):286-95. doi: 10.3109/08982104.2011.563364. Epub 2011 Mar 18. J Liposome Res. 2011. PMID: 21417671
-
Optimization of methazolamide-loaded solid lipid nanoparticles for ophthalmic delivery using Box-Behnken design.J Liposome Res. 2014 Sep;24(3):171-81. doi: 10.3109/08982104.2014.891231. Epub 2014 Mar 10. J Liposome Res. 2014. PMID: 24611687 Review.
Cited by
-
Nanotechnology-enabled immunogenic cell death for improved cancer immunotherapy.Int J Pharm. 2023 Mar 5;634:122655. doi: 10.1016/j.ijpharm.2023.122655. Epub 2023 Jan 30. Int J Pharm. 2023. PMID: 36720448 Free PMC article. Review.
-
Synthesis and Characterization of Antibiotic-Loaded Biodegradable Citrate Functionalized Mesoporous Hydroxyapatite Nanocarriers as an Alternative Treatment for Bone Infections.Pharmaceutics. 2022 Apr 30;14(5):975. doi: 10.3390/pharmaceutics14050975. Pharmaceutics. 2022. PMID: 35631561 Free PMC article.
References
-
- Jordan M., Wurm F. Transfection of adherent and suspended cells by calcium phosphate. Methods. 2004;33:136–143. - PubMed
-
- Uskoković V., Uskoković D.P. Nanosized hydroxyapatite and other calcium phosphates: chemistry of formation and application as drug and gene delivery agents. J Biomed Mater Res B Appl Biomater. 2011;96:152–191. - PubMed
-
- Chen Q., Wong C., Lu W. Strengthening mechanisms of bone bonding to crystalline hydroxyapatite in vivo. Biomaterials. 2004;25:4243–4254. - PubMed
-
- Oyane A., Wang X., Sogo Y. Calcium phosphate composite layers for surface-mediated gene transfer. Acta Biomater. 2012;8:2034–2046. - PubMed
-
- Pedraza C.E., Bassett D.C., McKee M.D. The importance of particle size and DNA condensation salt for calcium phosphate nanoparticle transfection. Biomaterials. 2008;29:3384–3392. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

