The role of serratiopeptidase in the resolution of inflammation
- PMID: 32104332
- PMCID: PMC7032259
- DOI: 10.1016/j.ajps.2017.01.003
The role of serratiopeptidase in the resolution of inflammation
Abstract
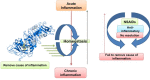
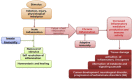
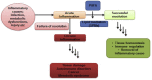
Inflammation remains a key event during most of the diseases and physiological imbalance. Acute inflammation is an essential physiological event by immune system for a protective measure to remove cause of inflammation and failure of resolution lead to chronic inflammation. Over a period of time, a number of drugs mostly chemical have been deployed to combat acute and chronic inflammation. Recently, enzyme based anti-inflammatory drugs became popular over conventional chemical based drugs. Serratiopeptidase, a proteolytic enzyme from trypsin family, possesses tremendous scope in combating inflammation. Serine protease possesses a higher affinity for cyclooxygenase (COX-I and COX-II), a key enzyme associated with production of different inflammatory mediators including interleukins (IL), prostaglandins (PGs) and thromboxane (TXs) etc. Currently, arthritis, sinusitis, bronchitis, fibrocystic breast disease, and carpal tunnel syndrome, etc. are the leading inflammatory disorders that affected the entire the globe. In order to conquer inflammation, both acute and chronic world, physician mostly relies on conventional drugs. The most common drugs to combat acute inflammation are Nonsteroidal anti-inflammatory drugs (NSAIDs) alone and or in combination with other drugs. However, during chronic inflammation, NSAIDs are often used with steroidal drugs such as autoimmune disorders. These drugs possess several limitations such as side effects, ADR, etc. In order to overcome these limitations and complications, enzyme based drugs (anti-inflammatory) emerged, and aim for a new high since the last decade. Serine protease, the largest proteolytic family has been reported for several therapeutic applications, including anti-inflammatory. Serratiopeptidase is a leading enzyme which has a very long history in medical as an effective anti-inflammatory drug. Current study emphasizes present scenario and future prospect of serratiopeptidase as an anti-inflammatory drug. The study also illustrates a comparative analysis of conventional drugs and enzyme based therapeutic to combat inflammation.
Keywords: ADR, adverse drug reaction; ALL, acute lymphoblastic leukemia; COX, cyclooxygenase; Cyclooxygenase; EC, enzyme commission; Enzyme therapeutics; IL, interleukins; Inflammation; LOX, lipoxygenase; NSAIDs; NSAIDs, non-steroidal anti-inflammatory drugs; PGs, prostaglandins; RA, rheumatoid arthritis; SPMs, specialized pro-resolvins mediators; Serratiopeptidase; Steroids; TXs, thromboxane; t-PA, tissue plasminogen activator.
© 2017 Shenyang Pharmaceutical University. Production and hosting by Elsevier B.V.
Figures

References
-
- Kang T.S., Stevens R.C. Structural aspects of therapeutic enzymes to treat metabolic disorders. Hum Mutat. 2009;30(12):1591–1610. - PubMed
-
- Valayannopoulos V., Brassier A., Chabli A. Enzyme replacement therapy for lysosomal storage disorders. Arch Pediatr. 2011;18(10):1119–1123. - PubMed
-
- Verma M.K., Pulicherla K.K. Enzyme promiscuity in Earthworm serine protease- Substrate versatility and therapeutic potential. Amino Acids. 2016;48(4):941–948. - PubMed
-
- Li Y., Cirino P.C. Recent advances in engineering proteins for biocatalysis. Biotechnol Bioeng. 2014;111(7):1273–1287. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

