Anti-CDCP1 immuno-conjugates for detection and inhibition of ovarian cancer
- PMID: 32104500
- PMCID: PMC7019151
- DOI: 10.7150/thno.30736
Anti-CDCP1 immuno-conjugates for detection and inhibition of ovarian cancer
Abstract
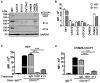
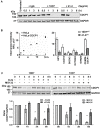
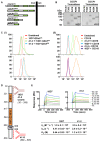
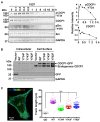
CUB-domain containing protein 1 (CDCP1) is a cancer associated cell surface protein that amplifies pro-tumorigenic signalling by other receptors including EGFR and HER2. Its potential as a cancer target is supported by studies showing that anti-CDCP1 antibodies inhibit cell migration and survival in vitro, and tumor growth and metastasis in vivo. Here we characterize two anti-CDCP1 antibodies, focusing on immuno-conjugates of one of these as a tool to detect and inhibit ovarian cancer. Methods: A panel of ovarian cancer cell lines was examined for cell surface expression of CDCP1 and loss of expression induced by anti-CDCP1 antibodies 10D7 and 41-2 using flow cytometry and Western blot analysis. Surface plasmon resonance analysis and examination of truncation mutants was used to analyse the binding properties of the antibodies for CDCP1. Live-cell spinning-disk confocal microscopy of GFP-tagged CDCP1 was used to track internalization and intracellular trafficking of CDCP1/antibody complexes. In vivo, zirconium 89-labelled 10D7 was detected by positron-emission tomography imaging, of an ovarian cancer patient-derived xenograft grown intraperitoneally in mice. The efficacy of cytotoxin-conjugated 10D7 was examined against ovarian cancer cells in vitro and in vivo. Results: Our data indicate that each antibody binds with high affinity to the extracellular domain of CDCP1 causing rapid internalization of the receptor/antibody complex and degradation of CDCP1 via processes mediated by the kinase Src. Highlighting the potential clinical utility of CDCP1, positron-emission tomography imaging, using zirconium 89-labelled 10D7, was able to detect subcutaneous and intraperitoneal xenograft ovarian cancers in mice, including small (diameter <3 mm) tumor deposits of an ovarian cancer patient-derived xenograft grown intraperitoneally in mice. Furthermore, cytotoxin-conjugated 10D7 was effective at inhibiting growth of CDCP1-expressing ovarian cancer cells in vitro and in vivo. Conclusions: These data demonstrate that CDCP1 internalizing antibodies have potential for killing and detection of CDCP1 expressing ovarian cancer cells.
Keywords: CDCP1; antibody; immuno-conjugate; ovarian cancer.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Miyazawa Y, Uekita T, Hiraoka N, Fujii S, Kosuge T, Kanai Y. et al. CUB domain-containing protein 1, a prognostic factor for human pancreatic cancers, promotes cell migration and extracellular matrix degradation. Cancer Res. 2010;70:5136–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

