Engineered Living Materials Based on Adhesin-Mediated Trapping of Programmable Cells
- PMID: 32105449
- PMCID: PMC7091533
- DOI: 10.1021/acssynbio.9b00404
Engineered Living Materials Based on Adhesin-Mediated Trapping of Programmable Cells
Abstract
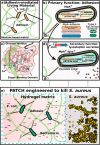
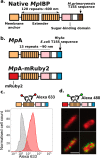
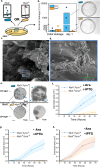
Engineered living materials have the potential for wide-ranging applications such as biosensing and treatment of diseases. Programmable cells provide the functional basis for living materials; however, their release into the environment raises numerous biosafety concerns. Current designs that limit the release of genetically engineered cells typically involve the fabrication of multilayer hybrid materials with submicrometer porous matrices. Nevertheless the stringent physical barriers limit the diffusion of macromolecules and therefore the repertoire of molecules available for actuation in response to communication signals between cells and their environment. Here, we engineer a novel living material entitled "Platform for Adhesin-mediated Trapping of Cells in Hydrogels" (PATCH). This technology is based on engineered E. coli that displays an adhesion protein derived from an Antarctic bacterium with a high affinity for glucose. The adhesin stably anchors E. coli in dextran-based hydrogels with large pore diameters (10-100 μm) and reduces the leakage of bacteria into the environment by up to 100-fold. As an application of PATCH, we engineered E. coli to secrete the bacteriocin lysostaphin which specifically kills Staphyloccocus aureus with low probability of raising antibiotic resistance. We demonstrated that living materials containing this lysostaphin-secreting E. coli inhibit the growth of S. aureus, including the strain resistant to methicillin (MRSA). Our tunable platform allows stable integration of programmable cells in dextran-based hydrogels without compromising free diffusion of macromolecules and could have potential applications in biotechnology and biomedicine.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Pardee K.; Slomovic S.; Nguyen P. Q.; Lee J. W.; Donghia N.; Burrill D.; Ferrante T.; McSorley F. R.; Furuta Y.; Vernet A.; Lewandowski M.; Boddy C. N.; Joshi N. S.; Collins J. J. (2016) Portable, On-Demand Biomolecular Manufacturing. Cell 167 (1), 248–259. e12.10.1016/j.cell.2016.09.013. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

