Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial
- PMID: 32105518
- PMCID: PMC8265386
- DOI: 10.1200/JCO.19.02274
Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial
Abstract
Purpose: Preoperative chemoradiotherapy may improve the radical resection rate for resectable or borderline resectable pancreatic cancer, but the overall benefit is unproven.
Patients and methods: In this randomized phase III trial in 16 centers, patients with resectable or borderline resectable pancreatic cancer were randomly assigned to receive preoperative chemoradiotherapy, which consisted of 3 courses of gemcitabine, the second combined with 15 × 2.4 Gy radiotherapy, followed by surgery and 4 courses of adjuvant gemcitabine or to immediate surgery and 6 courses of adjuvant gemcitabine. The primary end point was overall survival by intention to treat.
Results: Between April 2013 and July 2017, 246 eligible patients were randomly assigned; 119 were assigned to preoperative chemoradiotherapy and 127 to immediate surgery. Median overall survival by intention to treat was 16.0 months with preoperative chemoradiotherapy and 14.3 months with immediate surgery (hazard ratio, 0.78; 95% CI, 0.58 to 1.05; P = .096). The resection rate was 61% and 72% (P = .058). The R0 resection rate was 71% (51 of 72) in patients who received preoperative chemoradiotherapy and 40% (37 of 92) in patients assigned to immediate surgery (P < .001). Preoperative chemoradiotherapy was associated with significantly better disease-free survival and locoregional failure-free interval as well as with significantly lower rates of pathologic lymph nodes, perineural invasion, and venous invasion. Survival analysis of patients who underwent tumor resection and started adjuvant chemotherapy showed improved survival with preoperative chemoradiotherapy (35.2 v 19.8 months; P = .029). The proportion of patients who suffered serious adverse events was 52% versus 41% (P = .096).
Conclusion: Preoperative chemoradiotherapy for resectable or borderline resectable pancreatic cancer did not show a significant overall survival benefit. Although the outcomes of the secondary end points and predefined subgroup analyses suggest an advantage of the neoadjuvant approach, additional evidence is required.
Figures
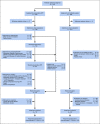
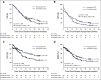

Comment in
-
Neoadjuvant or Adjuvant Therapy for Resectable or Borderline Resectable Pancreatic Cancer: Which Is Preferred?J Clin Oncol. 2020 Jun 1;38(16):1757-1759. doi: 10.1200/JCO.19.03318. Epub 2020 Mar 2. J Clin Oncol. 2020. PMID: 32119598 Free PMC article. No abstract available.
-
Time to Reconsider Staging Laparoscopy in Pancreatic Cancer?J Clin Oncol. 2020 Sep 1;38(25):2944-2945. doi: 10.1200/JCO.20.00996. Epub 2020 Jun 23. J Clin Oncol. 2020. PMID: 32574115 No abstract available.
-
Reply to S. Shi et al and G.W. Peters et al.J Clin Oncol. 2020 Sep 1;38(25):2945-2946. doi: 10.1200/JCO.20.01224. Epub 2020 Jun 23. J Clin Oncol. 2020. PMID: 32574116 No abstract available.
-
Neoadjuvant Treatment for Pancreatic Cancer: Still a Controversial Issue?J Clin Oncol. 2020 Sep 1;38(25):2943-2944. doi: 10.1200/JCO.20.00631. Epub 2020 Jun 23. J Clin Oncol. 2020. PMID: 32574118 No abstract available.
-
Randomized Trials for Esophageal, Liver, Pancreas, and Rectal Cancers.Int J Radiat Oncol Biol Phys. 2021 Feb 1;109(2):305-311. doi: 10.1016/j.ijrobp.2020.09.033. Int J Radiat Oncol Biol Phys. 2021. PMID: 33422270 No abstract available.
References
-
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. : Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 389:1011-1024, 2017 - PubMed
-
- Conroy T, Hammel P, Hebbar M, et al. : FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 379:2395-2406, 2018 - PubMed
-
- Merkow RP, Bilimoria KY, Tomlinson JS, et al. : Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg 260:372-377, 2014 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

