Point-of-care HIV viral load testing combined with task shifting to improve treatment outcomes (STREAM): findings from an open-label, non-inferiority, randomised controlled trial
- PMID: 32105625
- PMCID: PMC7183312
- DOI: 10.1016/S2352-3018(19)30402-3
Point-of-care HIV viral load testing combined with task shifting to improve treatment outcomes (STREAM): findings from an open-label, non-inferiority, randomised controlled trial
Abstract
Background: Monitoring HIV treatment with laboratory testing introduces delays for providing appropriate care in resource-limited settings. The aim of our study was to determine whether point-of-care HIV viral load testing with task shifting changed treatment and care outcomes for adults on antiretroviral therapy (ART) when compared with standard laboratory viral load testing.
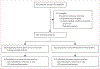
Methods: We did an open-label, non-inferiority, randomised controlled trial in a public clinic in Durban, South Africa. We enrolled HIV-positive adults (aged ≥18 years) who presented for their first routine HIV viral load test 6 months after ART initiation. Individuals were randomly assigned by a random number allocation sequence to receive either point-of-care viral load testing at enrolment and after 6 months with task shifting to enrolled nurses (intervention group), or laboratory viral load testing (standard-of-care group). The primary outcome was combined viral suppression (<200 copies per mL) and retention at 12 months after enrolment. A non-inferiority margin of 10% was used. Analysis was done by intention to treat. This study was registered with ClinicalTrials.gov, NCT03066128.
Findings: Between Feb 24, 2017, and Aug 23, 2017, we screened 657 participants, and 390 were enrolled and randomly assigned to either the intervention group (n=195) or standard-of-care group (n=195). 175 (90%) individuals in the intervention group and 148 (76%) individuals in the standard-of-care group had the primary outcome of retention with viral suppression, a difference of 13·9% (95% CI 6·4-21·2; p<0·00040). 182 participants (93%) in the intervention group had viral suppression compared with 162 (83%) in the standard-of-care group (difference 10·3%, 3·9-16·8; p=0·0025); 180 (92%) and 162 (85%) were retained in care (7·7%, 1·3-14·2; p=0·026). There were no adverse events related to point-of-care HIV viral load testing or task shifting.
Interpretation: Point-of-care viral load testing combined with task shifting significantly improved viral suppression and retention in HIV care. Point-of-care testing can simplify treatment and improve outcomes for HIV-positive adults receiving ART in resource-limited settings.
Funding: National Institute of Allergy and Infectious Diseases.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
All other authors declare no competing interests.
Figures
Comment in
-
Point-of-care HIV testing can help achieve UNAIDS targets.Lancet HIV. 2020 Apr;7(4):e216-e217. doi: 10.1016/S2352-3018(20)30043-6. Epub 2020 Feb 24. Lancet HIV. 2020. PMID: 32105624 No abstract available.
-
Conceptualising implementation strategies in HIV research.Lancet HIV. 2020 Jun;7(6):e382. doi: 10.1016/S2352-3018(20)30142-9. Lancet HIV. 2020. PMID: 32504570 No abstract available.
References
-
- Joint United Nations Programme on HIV/AIDS. Miles to go: closing gaps, breaking barriers, righting injustices. 2018. http://www.unaids.org/sites/default/files/media_asset/miles-to-go_en.pdf (accessed May 30, 2019).
-
- Joint United Nations Programme on HIV/AIDS. 90–90–90. An ambitious treatment target to help end the AIDS epidemic. 2014. http://www.unaids.org/sites/default/files/media_asset/90–90–90_en.pdf (accessed May 30, 2019).
-
- WHO. Interim technical update: technical and operational considerations for implementing HIV viral load testing. July 2014 https://www.who.int/hiv/pub/arv/viral-load-testing-technical-update/en/ (accessed Jan 29, 2020).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical