Protein X-ray Crystallography and Drug Discovery
- PMID: 32106588
- PMCID: PMC7179213
- DOI: 10.3390/molecules25051030
Protein X-ray Crystallography and Drug Discovery
Abstract
With the advent of structural biology in the drug discovery process, medicinal chemists gained the opportunity to use detailed structural information in order to progress screening hits into leads or drug candidates. X-ray crystallography has proven to be an invaluable tool in this respect, as it is able to provide exquisitely comprehensive structural information about the interaction of a ligand with a pharmacological target. As fragment-based drug discovery emerged in the recent years, X-ray crystallography has also become a powerful screening technology, able to provide structural information on complexes involving low-molecular weight compounds, despite weak binding affinities. Given the low numbers of compounds needed in a fragment library, compared to the hundreds of thousand usually present in drug-like compound libraries, it now becomes feasible to screen a whole fragment library using X-ray crystallography, providing a wealth of structural details that will fuel the fragment to drug process. Here, we review theoretical and practical aspects as well as the pros and cons of using X-ray crystallography in the drug discovery process.
Keywords: X-ray crystallography; drug discovery; high resolution; ligand screening; protein-ligand complexes; structure-based drug design; therapeutic targets; three-dimensional structures.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
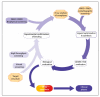
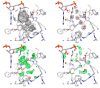
References
-
- Bernal J.D., Crowfoot D. X-ray photographs of crystalline pepsin. Nature. 1934;133:794–795. doi: 10.1038/133794b0. - DOI
-
- Clark G.L., Corrigan K.E. The crystal structure of insulin. Phys. Rev. 1932;40:639. doi: 10.1103/PhysRev.40.639. - DOI
-
- Hodgkin D.C. The X-ray analysis of the structure of penicillin. Adv. Sci. 1949;6:85–89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

