In vivo antiaging effects of alkaline water supplementation
- PMID: 32106720
- PMCID: PMC7054916
- DOI: 10.1080/14756366.2020.1733547
In vivo antiaging effects of alkaline water supplementation
Abstract
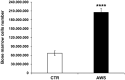
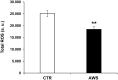
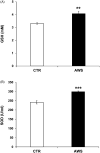
Telomeres length and telomerase activity are currently considered aging molecular stigmata. Water is a major requirement for our body and water should be alkaline. Recent reports have shown that aging is related to a reduced water intake. We wanted to investigate the effect of the daily intake of alkaline water on the molecular hallmark of aging and the anti-oxidant response. We watered a mouse model of aging with or without alkaline supplementation. After 10 months, we obtained the blood, the bone marrow and the ovaries from both groups. In the blood, we measured the levels of ROS, SOD-1, GSH, and the telomerase activity and analysed the bone marrow and the ovaries for the telomeres length. We found reduced ROS levels and increased SOD-1, GSH, telomerase activity and telomeres length in alkaline supplemented mice. We show here that watering by using alkaline water supplementation highly improves aging at the molecular level.
Keywords: Alkaline water supplementation; antiaging; antioxidant effect; telomerase; telomeres length.
Conflict of interest statement
The authors report no conflict of interest.
Figures





References
-
- Galloway A. The evolutionary biology of aging. By Michael R. Rose. New York: Oxford University Press. 1991. ix + 221 pp. ISBN 0-19-506133-0. $35.00 (cloth). Am J Phys Anthropol 1993;91:260–2.
-
- Partridge L, Barton NH.. Optimally, mutation and the evolution of ageing. Nature 1993;362:305–11. - PubMed
-
- Shay JW, Roninson IB.. Hallmarks of senescence in carcinogenesis and cancer therapy. Oncogene 2004;23:2919–33. - PubMed
-
- Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet 2005;6:611–22. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
