Carfilzomib, cyclophosphamide and dexamethasone for newly diagnosed, high-risk myeloma patients not eligible for transplant: a pooled analysis of two studies
- PMID: 32107329
- PMCID: PMC8018137
- DOI: 10.3324/haematol.2019.243428
Carfilzomib, cyclophosphamide and dexamethasone for newly diagnosed, high-risk myeloma patients not eligible for transplant: a pooled analysis of two studies
Abstract
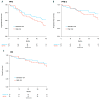
Despite remarkable advances in the treatment of multiple myeloma in the last decades, the prognosis of patients harboring high-risk cytogenetic abnormalities remains dismal as compared to that of standard-risk patients. Proteasome inhibitors demonstrated to partially ameliorate the prognosis of high-risk patients. We pooled together data from two phase I/II trials on transplant-ineligible patients with multiple myeloma receiving upfront carfilzomib cyclophosphamide and dexamethasone followed by carfilzomib maintenance. The aim of this analysis was to compare treatment outcomes in patients with standard- versus high-risk cytogenetic abnormalities detected by fluorescence in situ hybridization (FISH) analysis. High risk was defined by the presence of at least one chromosomal abnormality, including t(4;14), del17p and t(14;16). Overall, 94 patients were included in the analysis: 57 (61%) in the standard-risk and 37 (39%) in the high-risk group. Median follow-up was 38 months. In standard- vs. high-risk patients, we observed similar progression-free survival (3-year PFS: 52% vs. 43%, respectively; p=0.50), overall survival (3-year OS: 78% vs. 73%; p=0.38), and overall response rate (88% vs 95%; p=0.47), with no statistical differences between the two groups. No difference in terms of progression-free survival was observed between patients with or without del17p. Carfilzomib, used both as induction and maintenance agent for transplant-ineligible newly diagnosed multiple myeloma patients, mitigated the poor prognosis carried by high-risk cytogenetics and resulted into similar progression-free survival and overall survival, as compared to standard-risk patients. ClinicalTrials.gov IDs: NCT01857115 (IST-CAR-561) and NCT01346787 (IST-CAR-506).
Figures
References
-
- Greipp PR, San Miguel J, Durie BGM, et al. . International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412-3420. - PubMed
-
- Larocca A, Dold SM, Zweegman S, et al. . Patient-centered practice in elderly myeloma patients: an overview and consensus from the European Myeloma Network (EMN). Leukemia. 2018;32(8):1697-1712. - PubMed
-
- Moreau P, Attal M, Garban F, et al. . Heterogeneity of t(4;14) in multiple myeloma. Long-term follow-up of 100 cases treated with tandem transplantation in IFM99 trials. Leukemia. 2007;21(9):2020-2024. - PubMed