Gene therapy conversion of striatal astrocytes into GABAergic neurons in mouse models of Huntington's disease
- PMID: 32107381
- PMCID: PMC7046613
- DOI: 10.1038/s41467-020-14855-3
Gene therapy conversion of striatal astrocytes into GABAergic neurons in mouse models of Huntington's disease
Abstract
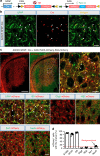
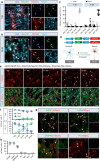
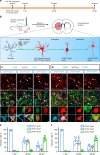
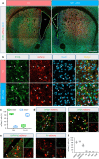
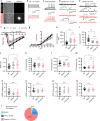
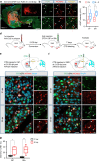
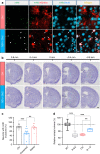
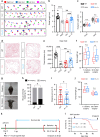
Huntington's disease (HD) is caused by Huntingtin (Htt) gene mutation resulting in the loss of striatal GABAergic neurons and motor functional deficits. We report here an in vivo cell conversion technology to reprogram striatal astrocytes into GABAergic neurons in both R6/2 and YAC128 HD mouse models through AAV-mediated ectopic expression of NeuroD1 and Dlx2 transcription factors. We found that the astrocyte-to-neuron (AtN) conversion rate reached 80% in the striatum and >50% of the converted neurons were DARPP32+ medium spiny neurons. The striatal astrocyte-converted neurons showed action potentials and synaptic events, and projected their axons to the targeted globus pallidus and substantia nigra in a time-dependent manner. Behavioral analyses found that NeuroD1 and Dlx2-treated R6/2 mice showed a significant extension of life span and improvement of motor functions. This study demonstrates that in vivo AtN conversion may be a disease-modifying gene therapy to treat HD and other neurodegenerative disorders.
Conflict of interest statement
Gong Chen is a co-founder of NeuExcell Therapeutics Inc.
Figures
References
-
- Bates, G. P. et al. Huntington disease. Nat. Rev. Dis. Primers1, 15005 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

